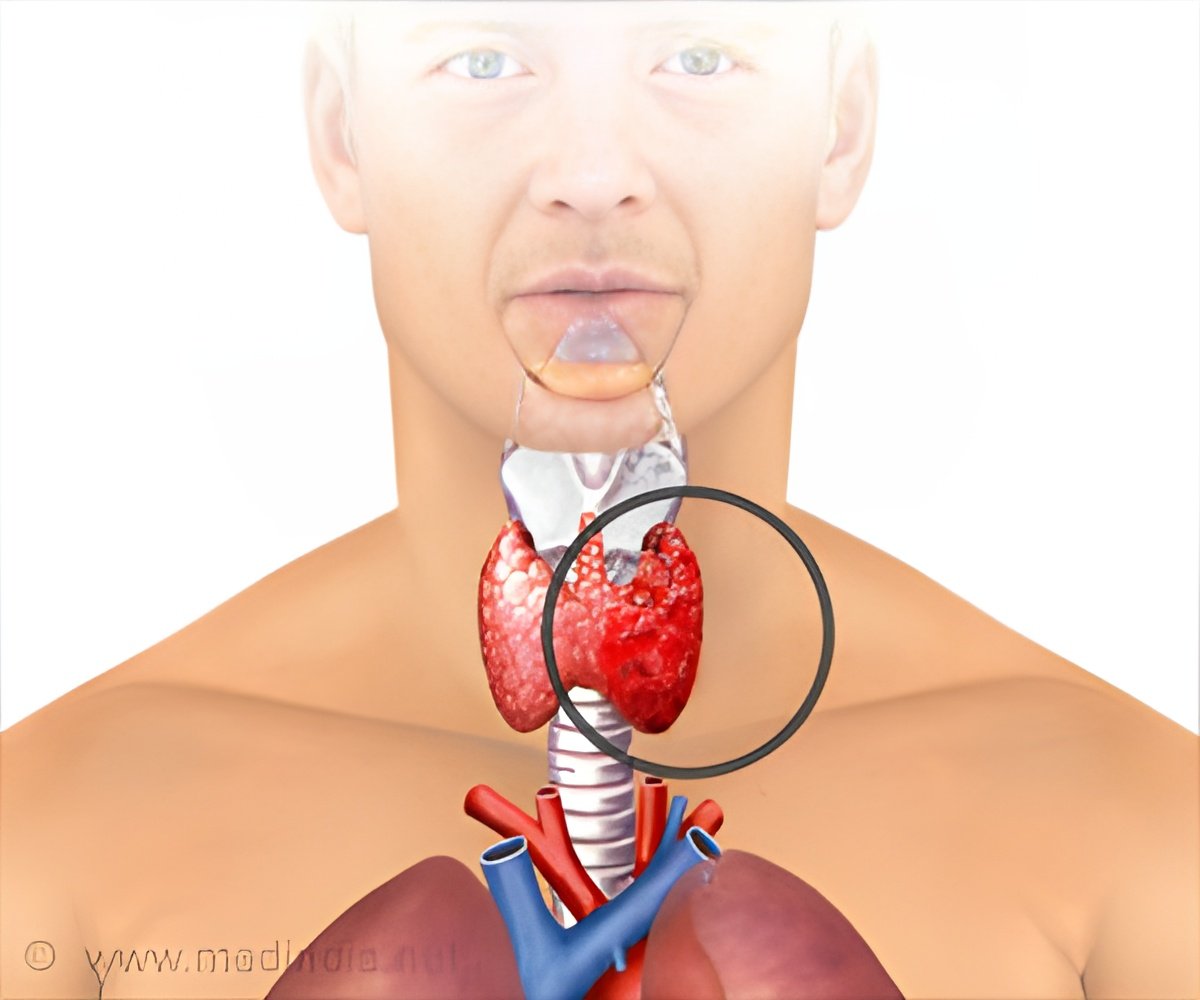
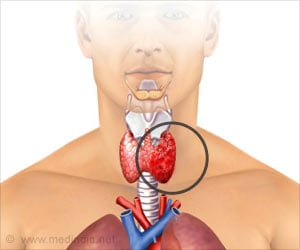
The BRAF mutations that occur in 40 to 50 percent of thyroid cancer patients are linked to aggressive tumors and reduced ability of tumors to respond to radioactive iodine.
In the current advancement in medicine, genetic mutations that cause cancer are targeted rather than the tumor. This continues to be a successful approach for some patients. Researchers from the Penn Medicine and the other institutions have found that treating metastatic thyroid cancer patients harboring a BRAF mutation with the targeted therapy vemurafenib has shown promising anti-tumor activity in a third of patients. Vemurafenib was originally approved for melanoma patients with the mutation. //
The phase II clinical study included results from 51 patients enrolled at 10 centers around the world with progressive, radioactive iodine-refractory (RAI) papillary thyroid cancer and a BRAF mutation who were no longer responding to prior therapies. After a 15-month follow-up, 16 patients from two cohorts had partial responses, with an overall response rate of 38 and 27 percent in each of the two cohorts.
TOP INSIGHT
The drug Vemurafenib has been shown to be effective to treat thyroid cancer patients with BARF mutation who do not respond to radioactive iodine.
"For this group of patients, who have little to no options, that's a significant improvement," said Marcia Brose, MD, PhD, an associate professor of Otorhinolaryngology: Head and Neck Surgery and Hematology/Oncology and director of the Center for Rare Cancers and Personalized Therapy and Penn's Abramson Cancer Center. "This promising clinical trial is the next step in a series of trials to identify new drugs that are fundamentally shifting the horizon - improving the outcome for patients with advanced differentiated thyroid cancer."
About 62,450 people were diagnosed with differentiated thyroid cancer in 2015. BRAF mutations, which occur in about 40 to 50 percent of these patients, have been associated with aggressive tumors and decreased the ability of tumors to respond to radioactive iodine, typically the first line of treatment in these patients. Patients are often cured by surgery, with or without RAI, but 50 percent of them with residual, recurrent or metastatic ultimately do not respond to RAI.
Vemurafenib joins other multi-targeted kinase inhibitors (MKIs) (sorafenib, lenvatinib) shown to be effective in this patient population; in spite of responses to these drugs, the responses are temporary and additional treatment options are needed.
For the study, researchers enrolled a total of 51 patients between January 2011 and January 2013. Patients in cohort one (26 patients) had not been previously treated with MKIs, while patients in the cohort (25) were treated with MKIs. Some in both groups had previously been treated with chemotherapy as well.
In cohort one, 10 patients had a partial response to vemurafenib, and an additional nine achieved stable disease for at least six months, for a combined disease control rate of 73 percent. The median progression-free survival was 18.2 months.
In cohort two, which had patients that were heavily pretreated, six patients had a partial response, and six achieved stable disease for at least six months, for a combined disease control rate of 54.5 percent. The median progression-free survival was 8.9 months and median overall survival was 14.4 months.
Overall, the side effects experienced by the patients were consistent with that of melanoma patients, except for the higher rates of weight loss, dysgeusia (distortion of taste), anemia (lack of iron), increased creatine levels, and hepatic laboratory abnormalities.
"Due to our prior successes in treating these patients with sorafenib and lenvatinib, patients are doing better, but they still ultimately progress, and we need additional agents with different mechanisms of action," Brose said. "Vemurafenib is the first non-VEGFR inhibitor to show activity in this patient population and as such is an important addition to our treatment options for these patients."
The results were published in this week's
Lancet Oncology.
Source-Eurekalert
 MEDINDIA
MEDINDIA

 Email
Email










