Three-dimensional cell cultures in the form of stars, diamonds and circles are generated by an exciting new digital microfluidics platform.
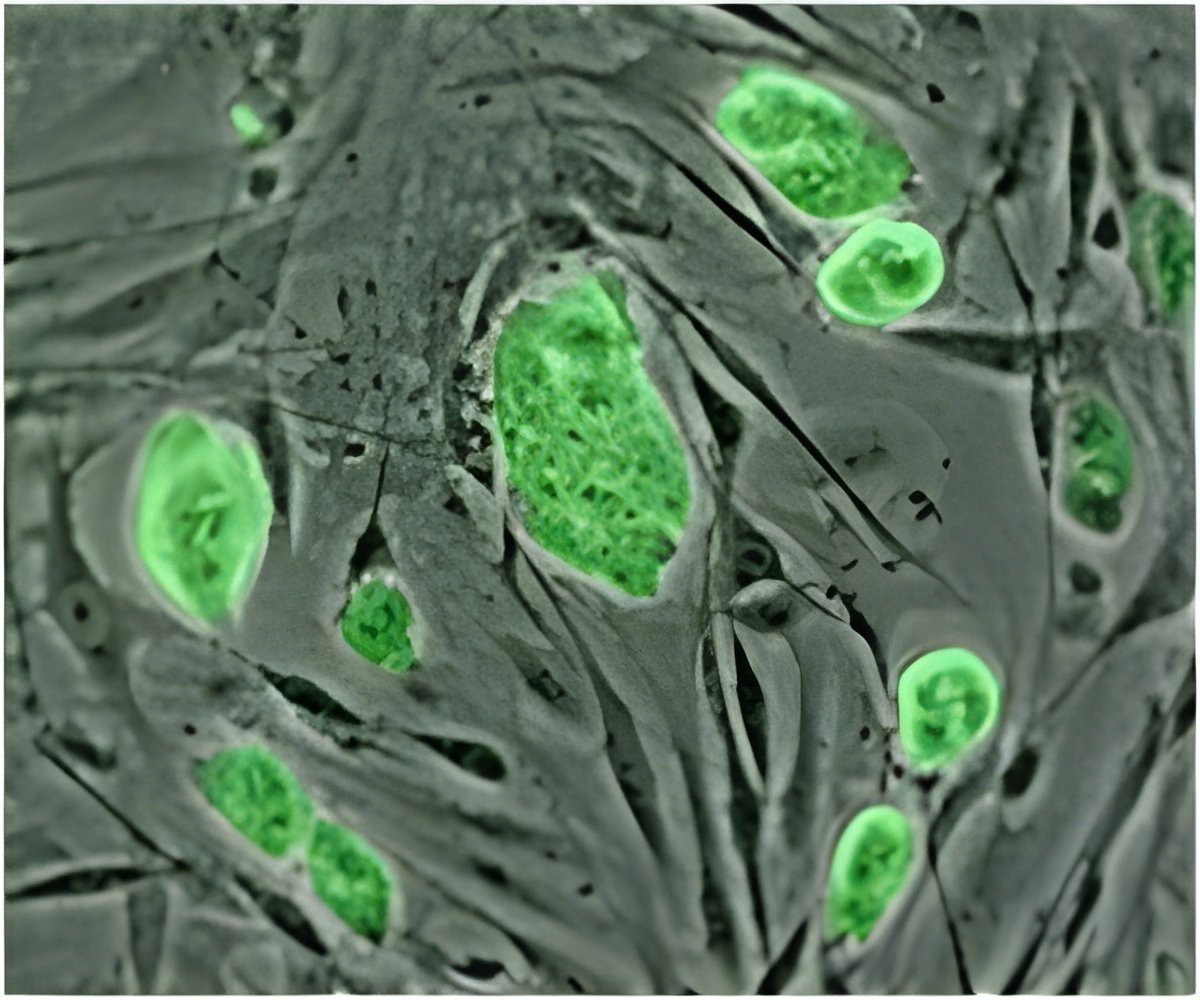
"We already know that the microenvironment can greatly influence cell fate," says Irwin A. Eydelnant, recent doctoral graduate from IBBME and first author of the publication. "The important part of this study is that we've developed a tool that will allow us to investigate the sensitivity of cells to their 3D environment."
"Everyone wants to do three-dimensional (3D) cell culture," explains Aaron Wheeler, Professor and Canada Research Chair in Bioanalytical Chemistry at the Institute of Biomaterials & Biomedical Engineering (IBBME), the Department of Chemistry, and the Donnelly Centre for Cellular and Biomolecular Research (DCCBR) at the University of Toronto. "Cells grown in this manner share much more in common with living systems than the standard two-dimensional (2D) cell culture format," says Wheeler, corresponding author of the study.
But more naturalistic, 3D cell cultures are a challenge to grow. "The reagents are expensive, the materials are inconvenient for automation, and 3D matrices break down upon repeated handling," explains Wheeler, who was named an Inventor of the Year by the University of Toronto in 2012.
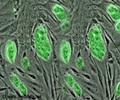
Eydelnant was able to address these difficulties by adapting a digital microfluidics platform first created in the Wheeler lab. Cells, caught up in a hydrogel material, are gently flowed across a small field that, on a screen, looks much like a tiny chessboard. The cells are strategically manipulated by a small electric field across a cutout shape on the top plate of the system, made from indium in oxide, and become fixed.
"When we grew kidney cells in these microgels, the cultures formed hollow sphere structures resembling primitive kidneys within four or five days," Eydelnant claims.
What's more, according to Eydelnant, the platform permits researchers to run, "32 experiments at the same time, automatically, and all on something the size of a credit card."
"We believe that this new tool will make 3D cell culture a more attractive and accessible format for cell biology research," he adds.
Although the researchers can foresee numerous possible applications for this platform, the team is "particularly excited" about its potential for personalized medicine.
Wheeler argues, "We may be able to collect small tissue samples from patients, distribute them into 3D gels on digital microfluidic devices, and screen for conditions to identify individually tailored therapies. This is in the 'dream' stages for now, but we think the methods described here will be useful for these types of applications in the future."
Source-Eurekalert
 MEDINDIA
MEDINDIA

 Email
Email