Nivolumab is an immune checkpoint inhibitor being tested in many cancers such as advanced melanoma and nonsmall cell lung cancer. It helps the body fight renegade cancer cells by restoring normal T-cell antitumor function. T-cells are a type of white blood cell that act like soldiers by searching out and destroying invaders. Nivolumab accomplishes this by suppressing an immune checkpoint modulator.
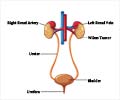
In this study, Nivolumab will be used in combination with sunitinib, pazopanib (existing treatments), or ipilimumab. These existing treatments have shown to reduce progression of the disease, but without lasting or durable response. Many patients eventually develop resistance to them. In other Phase 1 and 2 clinical trials, Nivolumab has shown durable responses in patients with mRCC.
The ASCO poster session describes an ongoing four-arm Phase 1 dose-escalation and expansion study evaluating combinations of Nivolumab with sunitinib, pazopanib, or ipilimumab in patients with mRCC. The primary objectives are to assess safety and tolerability, and to determine the recommended Phase 2 dose in patients with mRCC. The secondary objective is to assess preliminary antitumor activity. Researchers will also evaluate overall survival, pharmacodynamics, predictive biomarkers for the combinations, pharmacokinetics and immunogenicity of Nivolumab.
Source-Eurekalert