The National Institutes of Health defines a biomarker as "a characteristic that is objectively measured and evaluated as an indicator of normal biologic processes, pathogenic processes, or pharmacologic responses to a therapeutic intervention." In the past decade, there have been numerous biomarker discoveries, but most initially promising biomarkers have not been validated for clinical use.
To understand why so-called biomarker "breakthroughs" have not made it to the clinic, Eleftherios P. Diamandis, M.D., Ph.D., professor of pathology and laboratory medicine at Mount Sinai Hospital in Toronto and associate scientist at the Samuel Lunenfeld Research Institute of Mount Sinai Hospital reviewed some biomarkers initially hailed as breakthroughs and their subsequent failings.
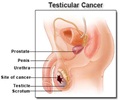
Diamandis first describes the requirements for biomarkers to be approved for clinical use: A biomarker must be released into circulation in easily detectable amounts by a small asymptomatic tumor or its micro-environment; and it should preferably be specific for the tissue of origin. Also, if the biomarker is affected by a non-cancer disease, its utility for cancer detection may be compromised. For example, the prostate-specific antigen (PSA) biomarker, which is used to detect prostate cancer, is also elevated in benign prostatic hyperplasia.
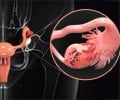
Diamandis looks at seven biomarkers that have emerged in the past 25 years, all of which were considered promising when they were first described. These include nuclear magnetic resonance of serum for cancer diagnosis; lysophosphatidic acid for ovarian cancer; four- and six-parameter diagnostic panels for ovarian cancer; osteopontin for ovarian cancer; early prostate cancer antigen-2 (EPCA-2) for prostate cancer detection; proteomic profiling of serum by mass spectrometry for ovarian cancer diagnosis; and peptidomic patterns for cancer diagnosis. Problems ranged from inappropriate statistical analysis to biases in case patient and control subject selection. For example, the problems with EPCA-2 included reporting values that were beyond the detection limit of the assay and using inappropriate reagents to test EPCA-2, such as solid surfaces coated with undiluted serum.
Diamandis concludes that "problems with pre-analytical, analytical, and post-analytical study design could lead to the generation of data that could be highly misleading."
Advertisement
Source-Eurekalert