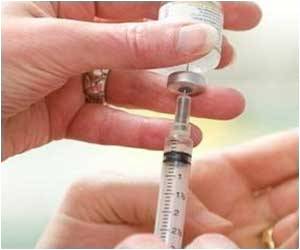
Merck was unable to get the nod from FDA to market Gardasil to women between the ages of 27 and 45.
Federal drug regulators held their ground and denied permission to Merck to use its cervical-cancer vaccine Gardasil in older women, as a protection against HPV or human papillomavirus. HPV can cause cervical cancer and genital warts.
The vaccine had received approval from FDA in 2006 for use in females between ages 9 to 26.
Source-Medindia