A promising 12-month follow-up data showing the world's first leadless pacemaker is demonstrating overall device performance, comparable to conventional pacemaker.
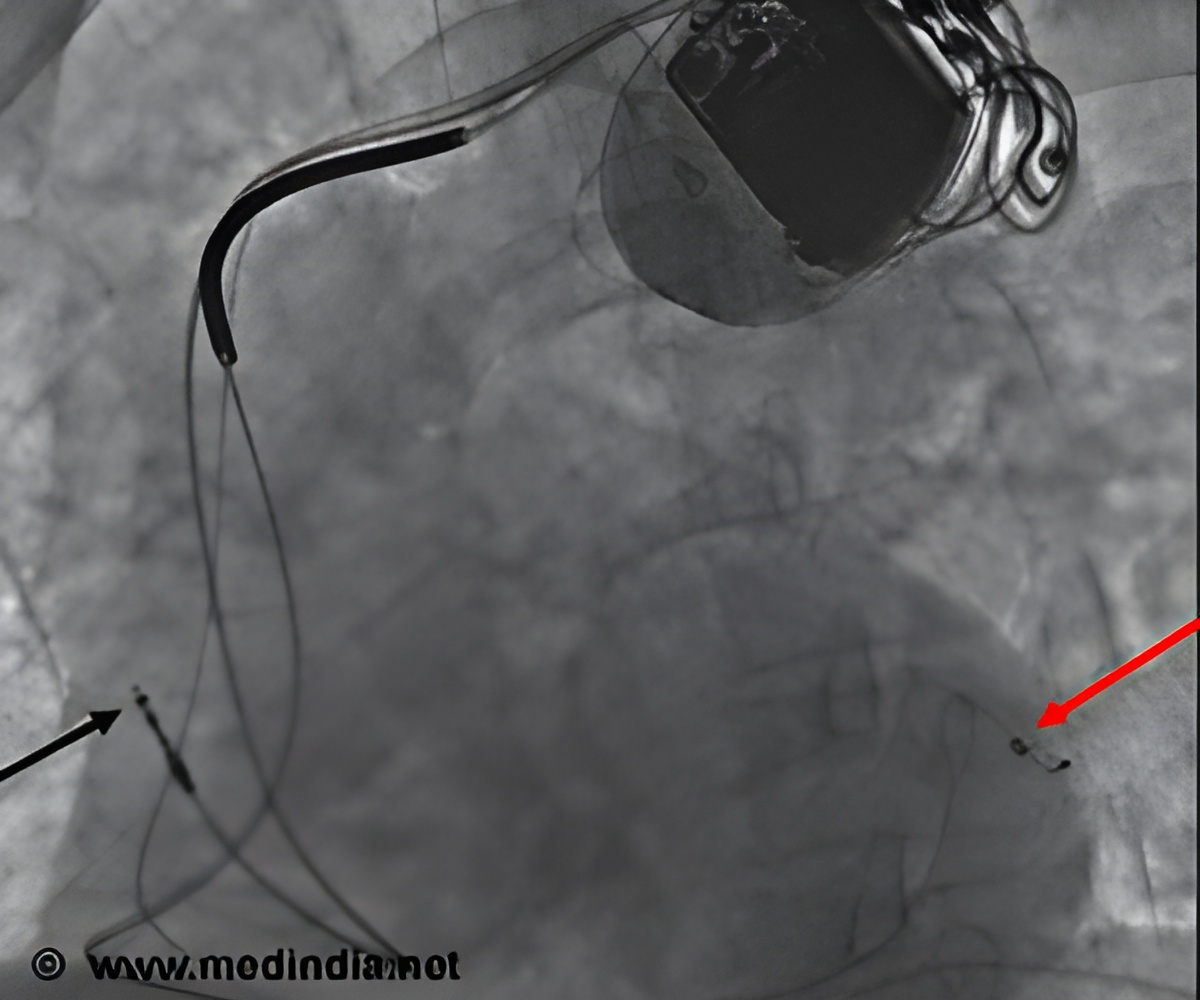
The LEADLESS study's long-term follow-up has evaluated 32 patients with a slowed heartbeat, bradycardia, who successfully received St. Jude Medical's Nanostim leadless pacemaker at two hospitals in Prague and one in Amsterdam.
The findings, which assessed device performance and patient outcomes through 12-months of follow-up, show pacing thresholds (0.43 volts) and sensing (10.32 mV) of the leadless pacemaker device are equivalent to those in traditional pacemakers. In addition, there was no experience of infections or failure to sense, pace, or communicate with the pacemaker.
"This is the first time we've seen one-year follow-up data for this innovative, wireless cardiac pacing technology and our results show the leadless pacemaker is comparable to traditional pacemakers," Dr. Vivek Reddy, Director of Arrhythmia Services at The Mount Sinai Hospital who is the study's co-investigator and Chairman of its Steering Committee, said.
"Our latest findings further support the promising performance and safety of this minimally-invasive, non-surgical pacing device. More long-term follow-up of these LEADLESS study patients will further our understanding of the potential advantages, benefits, and complication risks of leadless pacemaker technology, along with additional ongoing, larger trials," he said.
In February, Dr. Reddy was the first to implant the leadless pacemaker in the United States at The Mount Sinai Hospital launching the multicenter clinical trial LEADLESS II which aims to further test the leadless pacemaker more widely for safety and efficacy in 670 patients at 50 centers across the US and Canada.
Advertisement
The device, resembling a tiny, metal silver tube and smaller than a triple-A battery, is only a few centimeters in length, making it less than ten percent the size of a traditional pacemaker.
Advertisement
Source-ANI