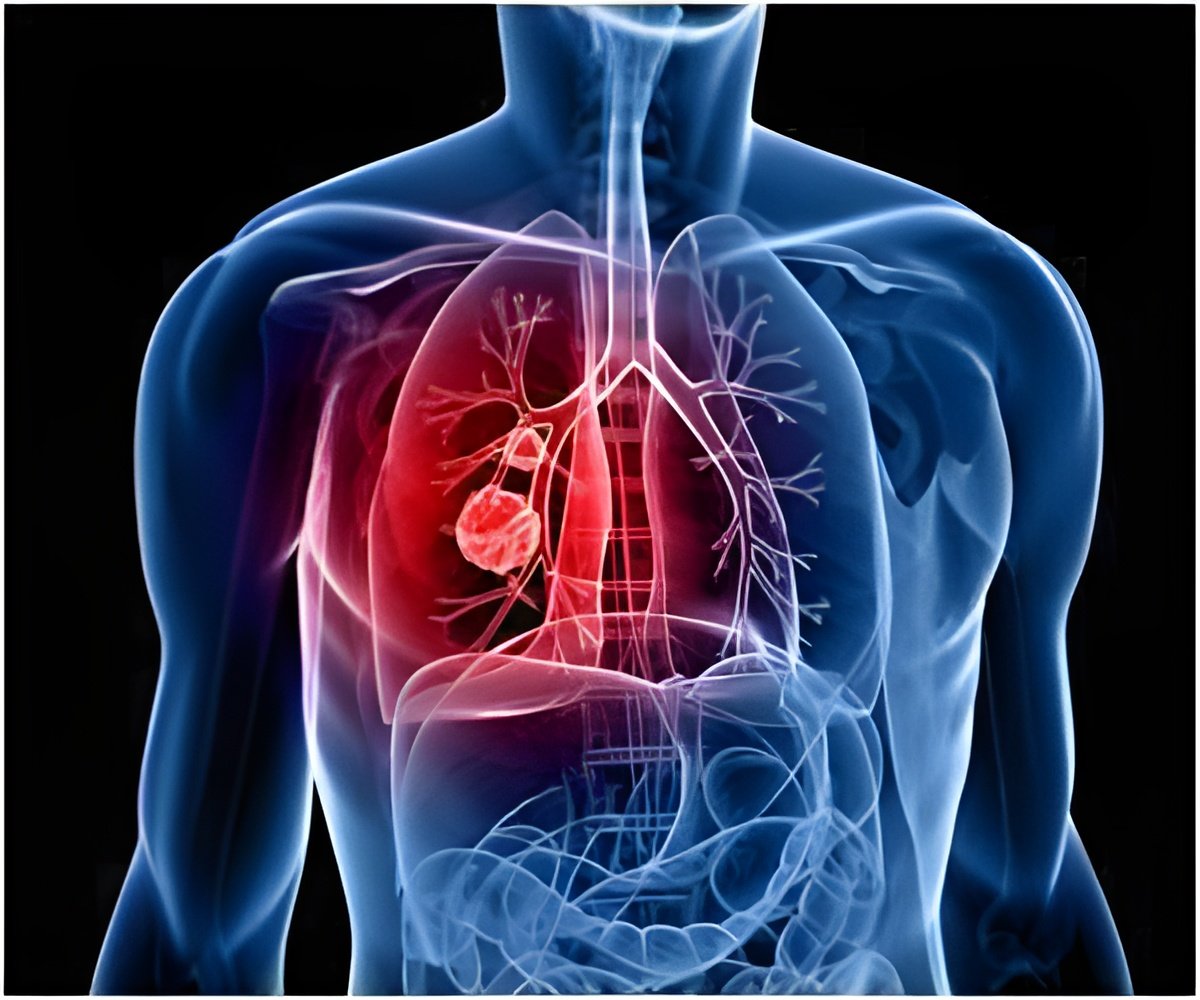
AstraZeneca revealed that it began late stage trials for its investigative MEK inhibitor selumetinib.
London-based pharmaceutical company AstraZeneca revealed that it began late stage trials for its investigative MEK inhibitor selumetinib to test its effectiveness as a potential treatment for non-small-cell lung cancer.
The company will enroll 634 NSCLC patients in the SELECT-1 (SELumetinib Evaluation as Combination Therapy-1) study. The study aims to compare the safety and efficacy of selumetinib in combination with Taxotere (Sanofi's version of docetaxel) to Taxotere and placebo. Progression free survival (PFS) and overall survival will be the main parameters in the study.
AZ has taken this step following encouraging results from phase II trials, where selumetinib plus Taxotere resulted in progression free survival of 5.3 months versus 2.1 months in the Taxotere alone arm.
Source-Medindia