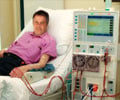
"Patients with advanced kidney disease are at highest risk for hyperkalemia thanks to a double whammy," says the study’s principal investigator, Matthew R. Weir, MD, professor of medicine and director of the Division of Nephrology at the University of Maryland School of Medicine. "Their kidneys are unable to remove potassium from the body effectively, and the patient may also be taking certain blood pressure control drugs that have been linked to high potassium levels. Current medications for hyperkalemia have gastrointestinal side effects that limit their extended use. We hoped the drug in this study would do the job with minimal side effects."
In this Phase 3 study of 237 patients with chronic kidney disease who were receiving RAAS inhibitors, 76 percent of the patients reached the target potassium level after four weeks on patiromer. Subsequently, 107 patients were randomly assigned to the drug or a placebo. The potassium level increase was greater in the placebo group than with patiromer, and elevated potassium recurred in 60 percent of the placebo group compared to 15 percent of the patiromer group through week eight. Mild-to-moderate constipation was the most common adverse event.
Source-Eurekalert