Ethyl Alcohol
Alcohol is a compound of carbon, hydrogen and oxygen. It is an organic compound with a hydroxyl (OH) group bound to a carbon atom of an alkyl or substituted alkyl group. It is formed when glucose is fermented by yeast. Generically, ethyl alcohol is called alcohol.
Ethyl alcohol is also known as ethanol,
Ethanol, in alcoholic beverages, is produced by
C6H12O6![]()
(carbohydrate) (ethanol)
The fermented grains or fruits are broken down to produce ethyl alcohol and carbon di oxide. This process of fermentation produces beverage alcohol. Ethanol has an energy value of seven kilocalories or twenty-nine joules per gram.
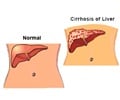
Ethanol is also produced for industrial purposes and finds use as a clean burning bio-fuel. Ethyl alcohol is sometimes adulterated with methyl alcohol or methanol. This is a more potent alcohol and is dangerous to consume as it gets broken down in the liver to form toxic compounds like formic acids and formaldehyde - the compounds can destroy the optic nerve leading to blindness. Other dangerous symptoms include - acidosis that may progress to death by respiratory failure.