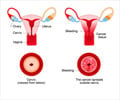
The Women In Government organization has undertaken a campaign to eliminate cervical cancer. This challenge was highlighted at the sixth International Multidisciplinary Congress of the European Research Organization on Genital Infection and Neoplasia (EUROGIN) held in Paris, France this week. The event featured prominent researchers and officials and advocates of public health from around the world.
The Women in Government is a non-profit, bi-partisan organization. Its president, Susan Crosby, presented the organization's initiative in fighting this preventable cancer. The organization was founded in 2004 and since then has introduced bills and resolutions targeting cervical cancer elimination in 45 states with it being enacted in 39 states.According to Ms Crosby the knowledge of the fact that cervical cancer is caused by the human papillomavirus, or HPV has led to the development of preventive vaccines. This in addition to proper diagnostic screening, such as HPV testing, cervical cancer can be eliminated. The organization’s however plans to ensure that women are educated about the need for screening. In addition access to all these sophisticated prevention strategies must be ensured regardless of socioeconomic status.
Ms. Crosby emphasized that legislative initiatives have included creating statewide cervical cancer elimination task forces, requiring health insurers to cover new FDA-approved technologies, and declaring statewide cervical cancer awareness days.
Cervical cancer has been claimed to be responsible for almost a quarter-million women deaths each year. The popularity of the Pap test has however helped to bring down cervical cancer rates in the United States over the last 60 years. The approval of a new screening test for HPV infections, which, when used in conjunction with a Pap is expected to increase the accuracy of the Pap to about100 percent.
An HPV vaccine is expected to receive approval from the FDA, which if widely used, could significantly decrease the number of cervical cancer cases worldwide.