The US Food and Drug Administration (FDA) is investigating Glaxo’s slimming drug Alli for liver damage.
Alli (orlistat 60mg) is an over-the-counter product that was approved by the FDA in 2007 to help promote weight loss when used together with a low-calorie, low-fat diet. During its first full year on the market in the United States, Alli generated $131 million in sales for GlaxoSmithKline and has been viewed as a very important product for phamaceutical company.On August 24, the FDA issued an Early Communication to notify the public that they are reviewing a potential liver damage risk with Alli and Xenical, a prescription version of the weight loss drug that contains 120 mg of orlistat.
The FDA said on its website that it is reviewing new safety information regarding reports of liver-related adverse events in patients taking orlistat.
“Xenical (orlistat 120mg) was approved as a prescription product by FDA in 1999 for obesity management in conjunction with a reduced caloric diet, and to reduce the risk of regaining weight after prior weight loss.
In 2007, Alli (orlistat 60mg) was approved for OTC use for weight loss in overweight adults, 18 years and older, in conjunction with a reduced-calorie and low-fat diet. Currently, orlistat is approved for marketing in approximately 100 countries. In January 2009, a nonprescription version of orlistat was approved for sale in the European Union.
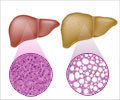
Between 1999 and October 2008, 32 reports of serious liver injury, including 6 cases of liver failure, in patients using orlistat were submitted to FDA’s Adverse Event Reporting System. Thirty of the 32 reports occurred outside the United States. The most commonly reported adverse events described in the 32 reports of serious liver injury were jaundice (yellowing of the skin or whites of the eyes), weakness, and abdominal pain. Hospitalization was reported in 27 of the 32 cases.
Advertisement
But FDA has clarified that it is not advising healthcare professionals to change their prescribing practices with orlistat. Consumers currently taking Xenical should continue to take it as prescribed and those using over-the-counter Alli should continue to use the product as directed.
Advertisement
This early communication is in keeping with FDA’s commitment to inform the public about its ongoing safety reviews of drugs. FDA will communicate its findings to the public as soon as its review of orlistat is complete.
But the drug manufacturers, GlaxoSmithKline (GSK) responded saying that it stands firmly behind the safety and efficacy of alli. Our primary priority is patient health, and we want people to know that there is no evidence that alli causes liver damage.
The FDA is reviewing data from suspected cases on liver injury associated with the use of orlistat/alli. Any routine assessment from a regulatory body does not mean that a risk or causal relationship exists.
Alli is a 'non-systemically' acting medicine - it is minimally absorbed in the blood andworks locally in the gastro-intestinal tract. There is therefore no obvious biological mechanism to suggest liver damage can occur with alli.
Liver changes can have many causes. People who are overweight and obese are predisposed to liver-related disorders.
The safety of consumers is of utmost importance to GSK. We continually monitor and evaluate reports of adverse effects associated with use of all of our products, including alli, the company stated.
Source-Medindia
GPL