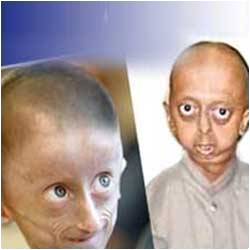
This clinical trial grew out of the identification of the defective progeria gene, LMNA, in 2003 through the Human Genome Project and the laboratory of current NIH Director Francis Collins. But the link to defective proteins called lamins that make up the envelope surrounding the cell nucleus came about through "untargeted" basic cell biology research. Veteran lamin researchers remember having their grant applications dismissed by review panels as "boring" and irrelevant. But basic work by Robert Goldman of the Northwestern University School of Medicine and other nuclear lamin researchers around the world revealed that a greasy tag molecule called farnesyl accumulates on defective Lamin A proteins, eventually warping the structure of the entire nuclear envelope and disrupting the orderly production of genetic messages in the nucleus that direct normal growth.
The identification of the defective LMNA gene transformed progeria into a "laminopathy," a now growing class of diseases caused by problems with the once-irrelevant nuclear lamins. "Normal" aging is thought to involve many of the same processes as laminopathies and gives this new clinical trial implications beyond progeria. With the discovery of the lamin link, clinical researchers were suddenly looking for farnesyl transferase inhibitors (FTI) for progeria treatment. They zeroed in on Lonafarnib, an FTI drug developed by Merck that had been extensively tested and found safe for use in adults and children but ineffective against its brain cancer targets. In the two and a half year clinical trial, physicians at Boston Children's gave Lonafarnib to 26 children with progeria.
Source-Eurekalert