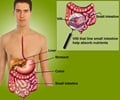
Alba/Teva’s larazotide acetate and Alvine/AbbVie’s latiglutenase, are the drugs which are anticipated to be launched in the US and the five European countries (France, Germany, Spain, Italy and the UK) by 2018 and 2019 respectively.
The drugs are targeted to patients who undergo gluten exposure while on a GFD equating to approximately 95% of the celiac disease patient population.
Abhilok Garg, GlobalData’s Analyst covering Immunology, says that the market will be driven primarily by the potential introduction of two novel pipeline drugs, Alba/Teva’s larazotide acetate and Alvine/AbbVie’s latiglutenase, which are designed to be used as adjunctive treatments to the current standard of care, a Gluten-Free Diet (GFD).
Garg comments: "Larazotide acetate is forecast to launch in the US in 2018, with the key competitive advantage of targeting the majority of the disease population, compared with the 4–7% of refractory patients, who are currently treated with generics including steroids and immunosuppressants."
Despite the anticipated impact of these novel drugs, there are a number of barriers to growth in the celiac disease treatment market, such as limited physician and patient awareness of the condition and a lack of confidence in pipeline drugs compared with a GFD.
Advertisement
Source-Medindia