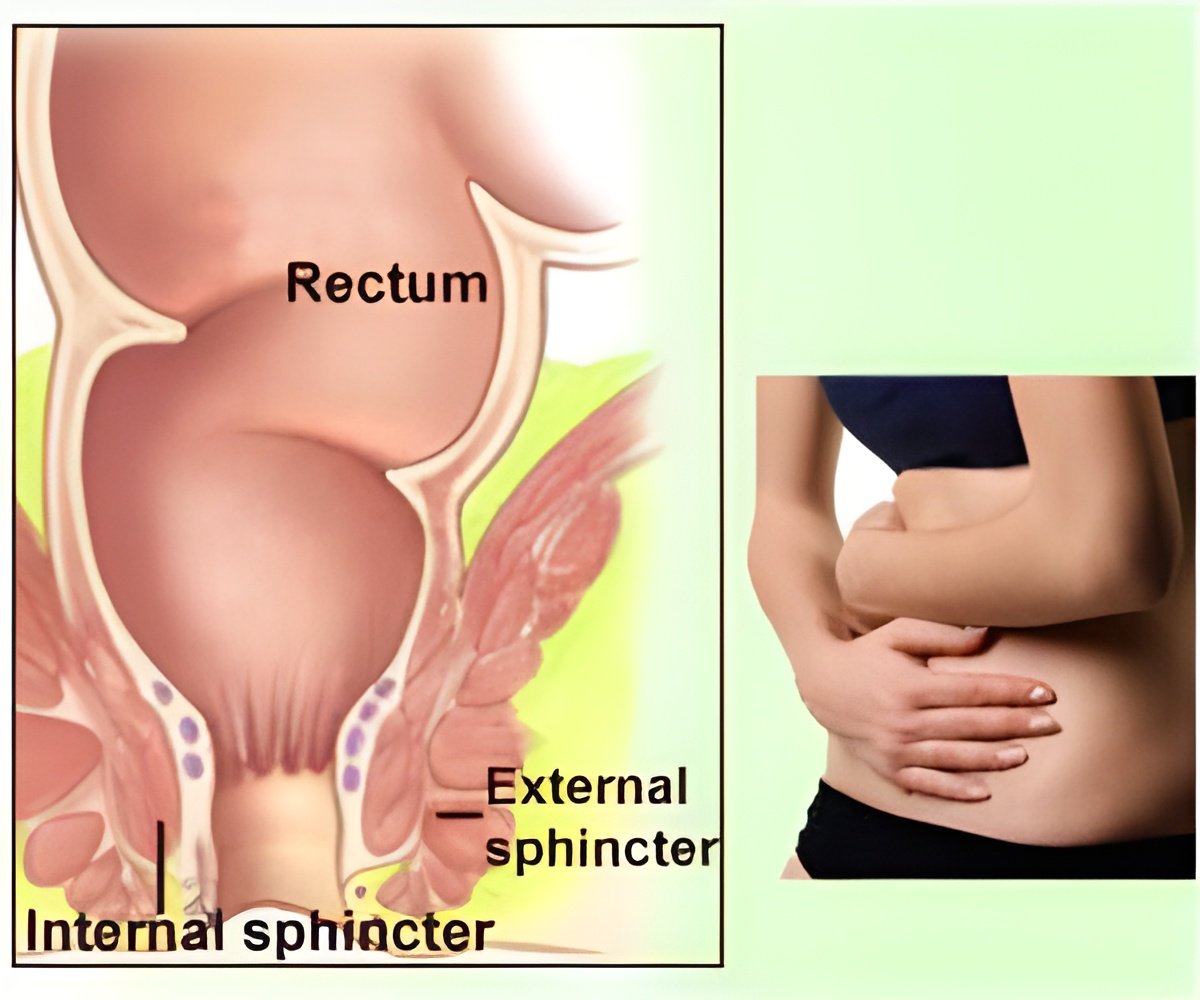
‘Torax Medical’s FENIX continence restoration system prevents involuntary discharge toward the other end of the gastrointestinal tract. The device is implanted using a single incision.’
Tweet it Now
However, the FENIX prevents involuntary discharge toward the other end of the gastrointestinal tract. The device is implanted using a single incision. The size of the bracelet is decided using a provided tool. X-rays are used to confirm the appropriate size of the implant and to make sure the functionality of the system following the procedure. Fecal incontinence or bowel incontinence is a lack of control over defecation, leading to involuntary loss of bowel contents - including flatus (gas), liquid stool elements and mucus, or solid feces. Fecal incontinence is a sign or a symptom, not a diagnosis.
Source-Medindia








