Roflumilast, a once-daily cream, showed significant improvements in patients with plaque psoriasis signs and symptoms in phase 2b trials, reveals a new study.
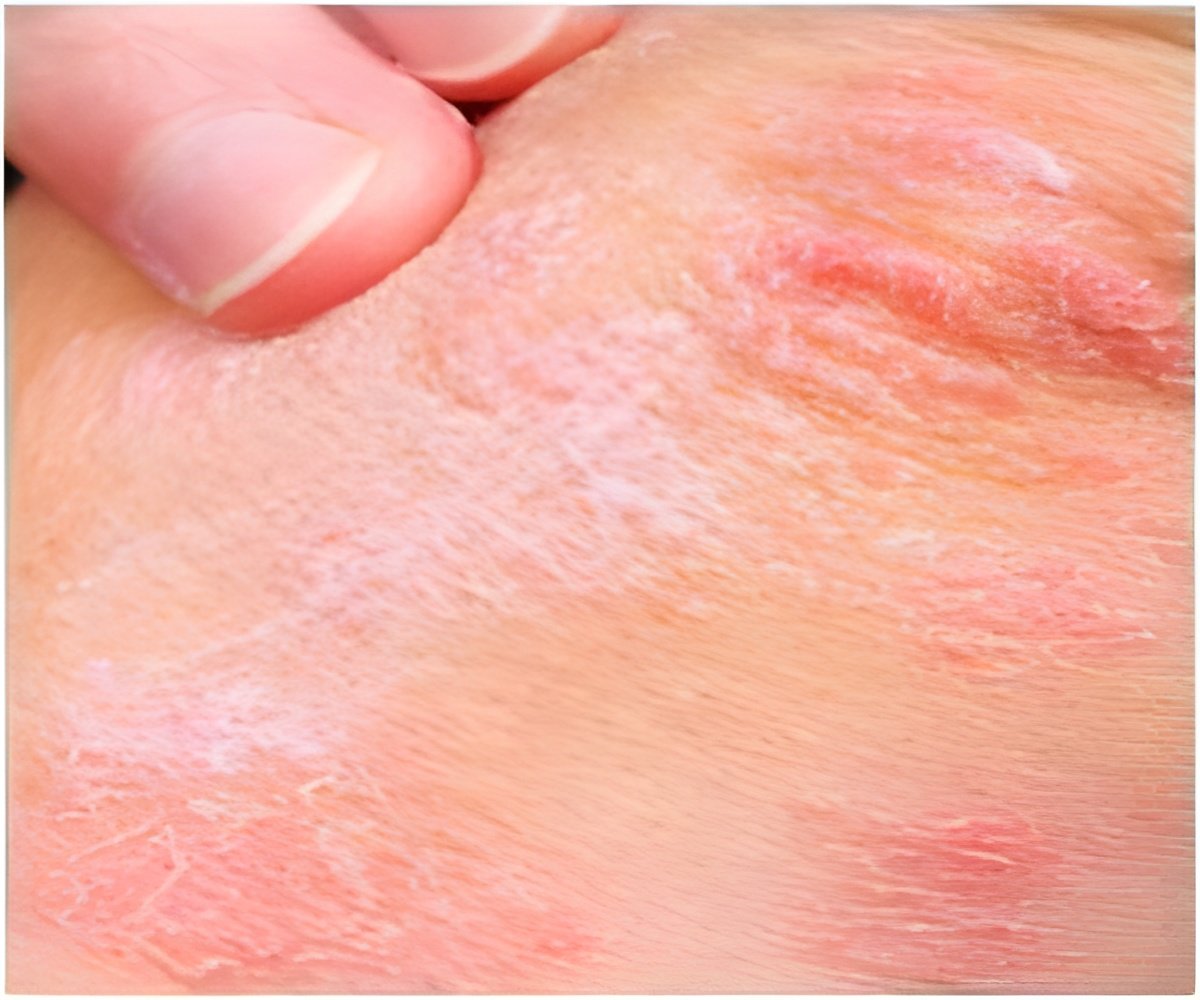
‘Plaque psoriasis imposes a significant burden on patients and is often linked to poor quality of life.
’
Read More..




“Effectively treating psoriasis with topical therapies, which the vast majority of patients receive, is especially challenging due to side effects, tolerability, or efficacy of existing topical treatments. Roflumilast cream could really be a game-changer.” Read More..
Roflumilast which contains PDE4, an intracellular enzyme that regulates pro-inflammatory and anti-inflammatory cytokine production and cell proliferation has been implicated in a wide range of inflammatory diseases and approved by the U.S. Food and Drug Administration for the treatment of chronic obstructive pulmonary disease (COPD) in 2011.
In the double-blinded trial, a total of 331 adults with plaque psoriasis were randomly assigned in a 1:1:1 ratio to receive topical treatment with roflumilast cream 0.3%, 0.15%, or matching treatment applied once daily for 12 weeks. A total of 109 patients were treated with roflumilast cream 0.3%, 113 with 0.15% cream, and 109 with the matching treatment. The primary efficacy outcome was global investigator assessment (IGA, a five-item scale assessing plaque thickening, scaling, and erythema, ranging from 0, clear, to 4, severe) of clear or almost clear at week six. Secondary outcomes included clear or almost clear plus a two-grade improvement (IGA success), IGA success on an intertriginous IGA, and change in psoriasis area and severity index (PASI).
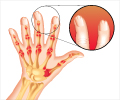
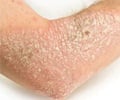
Psoriasis is an immune disease that occurs in about two percent of adults in Western countries. About 90 percent of psoriasis cases are plaque psoriasis, which is characterized by “plaques” or raised red areas of skin covered with a silver or white layer of scale. Psoriatic plaques can appear on any area of the body, but most often appear on the scalp, knees, elbows, trunk, and limbs, and the plaques are often itchy and sometimes painful. Plaques in certain anatomical areas present particular treatment challenges, including the face, elbows, knees, scalp, and other areas of the skin that may touch or rub together.
“The results from this phase 2b study data provide further evidence of the potential of roflumilast cream as a once-daily treatment for patients with plaque psoriasis who currently lack suitable treatment options,” said Howard Welgus, MD, Chief Medical Officer at Arcutis, the biopharmaceutical company, which funded and co-authored the study. “We are pleased to share these results, which suggest topical roflumilast was well tolerated and efficacious, with the broader scientific community.”
Advertisement
Source-Newswise