A new device designed to close a common heart defect known as a patent foramen ovale (PFO) is safe and effective at 90-days follow up, according to a new study released today at the Society for Cardiovascular Angiography and Interventions (SCAI) 32nd Annual Scientific Sessions in Las Vegas.
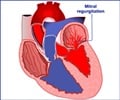
PFO is a common condition in which a hole that connects the two upper chambers of the heart (atria) during fetal development fails to close properly after birth. About 25 percent of the general population has PFO, which is associated with an increased risk of stroke. PFO may also be associated with migraines and decompression illness in divers.The FlatStent EF, developed by Coherex Medical,Inc., is unique because it is implanted within the PFO tunnel, leaving a minimal amount of the device exposed to circulation, which could reduce the risk of blood clots. The mechanism of action of the FlatStent EF should limit the incidence of other major complications associated with septal patching devices currently in use, such as erosion, persistent arrhythmia, and valve apparatus distortion.
In the study, 29 of 41 patients (70.7%) who received the FlatStent EF™ PFO Closure System had complete PFO closure immediately following implantation. After 90 days, 17 of 19 patients (89%) had complete or clinical closure. The remaining 22 patients had not yet completed the 90-day follow up exam.
"The FlatStent is designed to be safer and easier to use than current PFO closure systems," said Horst Sievert, MD, professor of internal medicine, cardiology and vascular medicine at the Cardiovascular Center in Frankfurt, Germany, and the study's principal investigator. "Given the number of patients living with PFO, the results of this study show promise in providing additional treatment options that could help prevent stroke and other conditions associated with PFO."
In the study, no device-related complications were reported. The 100-patient, multicenter registry study is designed to evaluate 30, 90 and 180-day safety and performance.
Source-Eurekalert
SRM