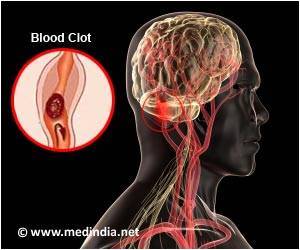
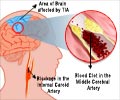
In the MR CLEAN (Multicenter Randomized Clinical trial of Endovascular treatment for Acute ischemic stroke in the Netherlands) comparative effectiveness study, intra-arterial treatment improved the rate of good functional outcome by 71 percent over treatment with medical management with tPA alone. Of patients who received intra-arterial treatment, 32.6% achieved a clinically defined positive stroke outcome compared with 19.1% of those who received medical management. Intra-arterial treatment was beneficial for all ages (18+), including the elderly (80+) - a population for whom intervention was previously thought to be risky. The trial began enrolling in 2010 and participating physicians used earlier generation intra-arterial technology. Currently available technology continues to improve upon the baseline set by MR CLEAN.
"Despite being the number one cause of long-term adult disability, stroke has been one of the most undertreated and devastating diseases in the world," said Albert J. Yoo, M.D., director of Acute Stroke Intervention in the Division of Interventional Neuroradiology/Endovascular Neurosurgery at Massachusetts General Hospital, assistant professor at Harvard Medical School and study author of MR CLEAN. "Now that intra-arterial treatment has been proven to provide significant benefits for stroke patients of all ages, we have a golden opportunity to dramatically improve the lives of stroke victims and, at the same time, help reduce costs in our healthcare system by returning patients to functional independence."
MR CLEAN also demonstrated:
- Intra-arterial treatment was effective for patients with the most common and devastating form of stroke, LVO (large vessel occlusion), for which tPA is less effective.
- Intra-arterial treatment is effective up to 6 hours after stroke onset vs. 3 to 4.5 hours for tPA.
- Both treatments were safe, with no differences relating to hemorrhage or mortality.
"The MR CLEAN study results provide evidence to support intra-arterial treatment in the fight against stroke and have the potential to change the standard of care," said Adam Elsesser, chief executive officer, director and founder, Penumbra. "Penumbra is proud to have supported the MR CLEAN trial, and these important results will aid our efforts to improve access to intra-arterial treatment for the millions of stroke patients around the world."
Penumbra provided an unrestricted grant for the trial, and its devices were among those included in the protocol. Companies providing grants had no influence over the trial's design or execution. Penumbra's newest intra-arterial treatment, which was not available at the time MR CLEAN was conducted, is the ACE™, a next generation clot extraction system that uses aspiration alone to engage and remove blood clots causing an acute ischemic stroke. Results with the Penumbra ACE system continue to improve upon the baseline set by MR CLEAN.
Advertisement