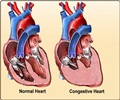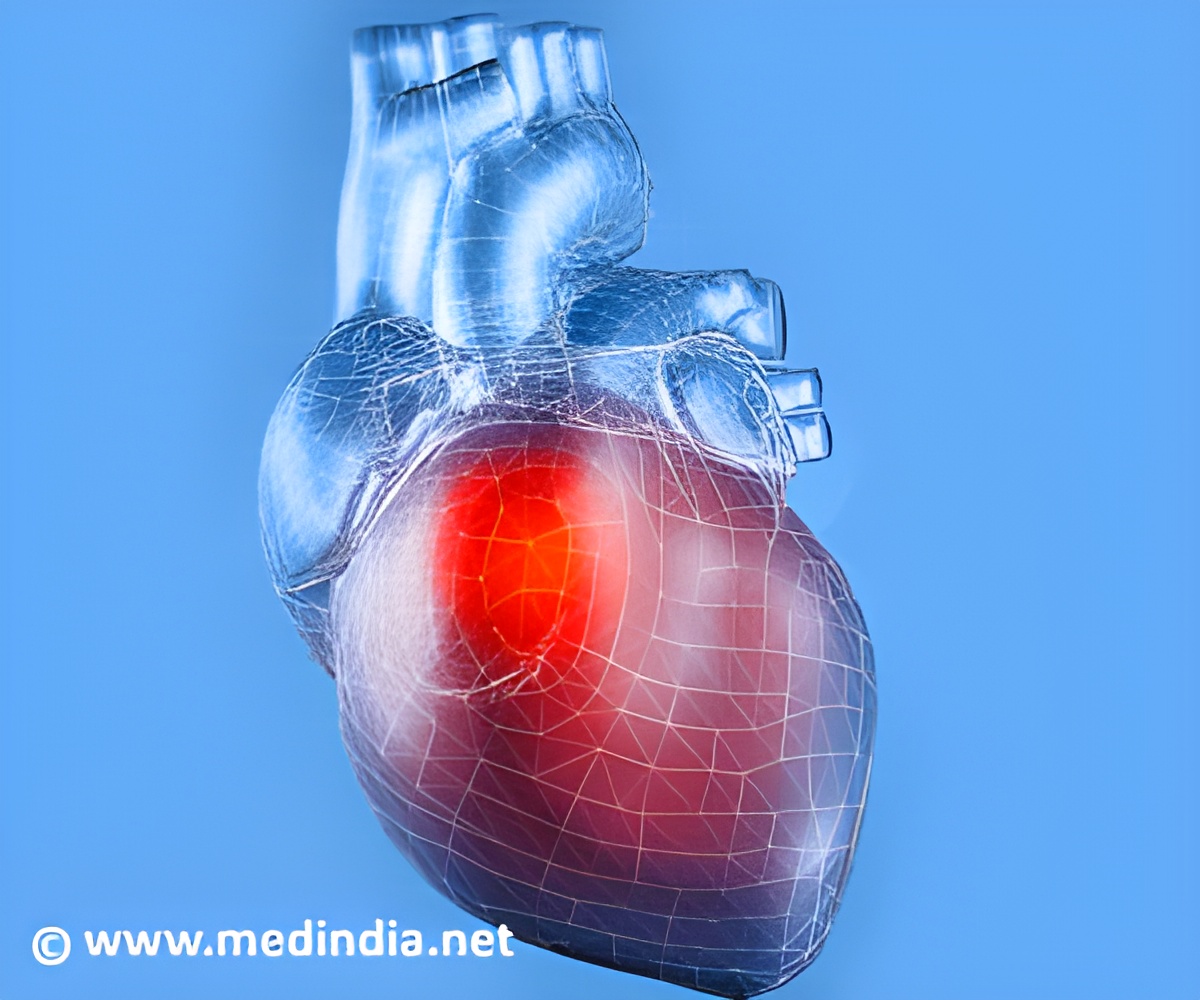
They will also come with low friction coating on the device can that reduces the friction between the device and leads.
“The goal for this innovative portfolio of devices is to bring implantable defibrillation reliability and patient safety to the next level. The new Ellipse ICD and Assura family of devices demonstrates St. Jude Medical’s commitment to developing technologies that provide physicians with advanced patient management tools to mitigate the most common ICD lead complications, especially those that can lead to ineffective high voltage therapy delivery”, the president of the St. Jude Medical Implantable Electronic Systems Division, Eric S. Fain said.
Source-Medindia