Risk of allergic reactions to HPV vaccination is considerable, but no major harm is envisaged, according to a study published in the Canadian Medical Association Journal (CMAJ).
In 2007, Australia implemented the National human papillomavirus (HPV) Vaccination Program, in an attempt to combat cervical caner.The programme provides quadrivalent HPV vaccine free to all women aged 12–26 years. Following notification of 7 presumptive cases of anaphylaxis in the state of New South Wales, Australia, researchers verified cases and compared the incidence of anaphylaxis following HPV vaccination to other vaccines in comparable settings.
The team contacted all patients with suspected anaphylaxis and obtained detailed histories from telephone interviews and a review of medical records
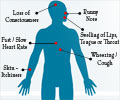
In a study of 114,000 women, they found 12 suspected of anaphylaxis, and confirmed 8 of these, in New South Wales. Symptoms included difficulty breathing, nausea and rashes.
The estimated rate of anaphylaxis following HPV vaccination was 2.6 per 100,000 doses administered compared with a rate 0.1 per 100 000 doses administered in a 2003 school-based meningococcal C vaccination program.
Overall then, the Gardasil shot is remarkably safe, declared doctors in an editorial accompanying the study in the Canadian Medical Association Journal. They did acknowledge the need to keep tabs on possible side effects, however.
Advertisement
Anaphylaxis is an acute systemic (multi-system) and severe Type I Hypersensitivity allergic reaction in humans and other mammals. Minute amounts of allergens may cause a life-threatening anaphylactic reaction. Anaphylaxis may occur after ingestion, skin contact, injection of an allergen or, in rare cases, inhalation.
Advertisement
Dr. Brotherton stresses "the importance of good training for staff administering vaccines in school or other settings in the recognition and management of suspected anaphylaxis and its reporting."
Anaphylaxis is a rare but serious adverse event and highlights the importance of vaccine safety studies after vaccine licensing and careful management of reactions in immunization clinics, says Dr. Neal Halsey, Institute of Vaccine Safety, Johns Hopkins Bloomberg School of Public Health in a related commentary.
He states "before concluding that the HPV vaccine is associated with higher rates of anaphylaxis than other vaccines everywhere, cases in other populations should be reviewed….As of July 21, 2008, 11 cases have been reported [in the US] in 2008. Over 13 million doses of this vaccine have been distributed as of the end of 2007."
A CMAJ editorial states that this study indicates the HPV vaccine is "remarkably safe." The study provides an excellent opportunity for Canada's public health community "to restart public discussions about the safety of the HPV vaccine, the precautions taken to mitigate risks if anaphylaxis occurs, and the care taken in surveillance for adverse events following vaccination," write Drs. Noni MacDonald, Matthew Stanbrook and Paul Hebert.
Source-Medindia
GPL