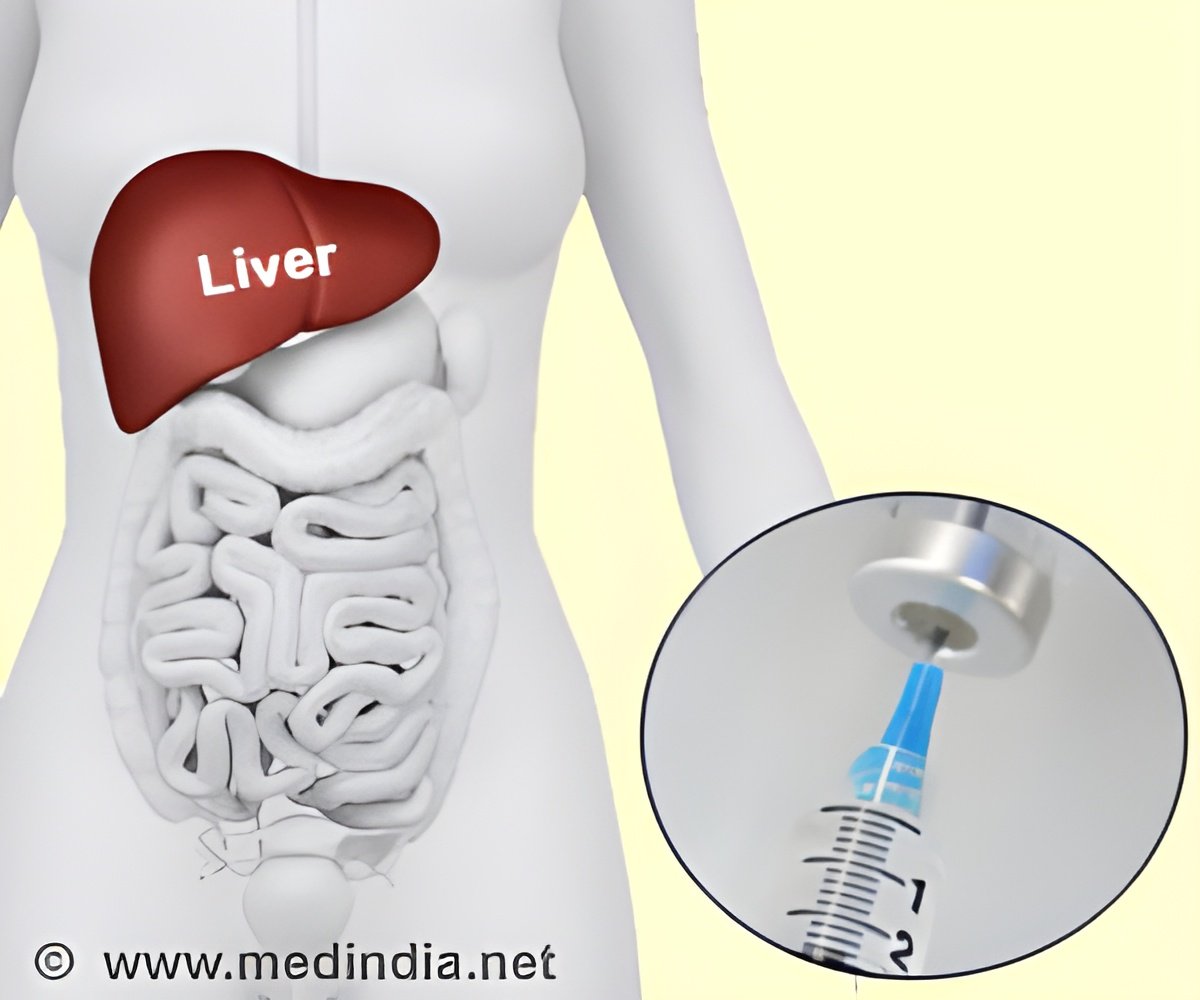
‘RG-101 in combination with a four week course of oral direct-acting antiviral treatment was well tolerated and resulted in high virologic response rates post-treatment among Hepatitis C.’
Tweet it Now
"These early results indicate the potential for RG-101 with oral DAA combination therapy to provide an effective Hepatitis C regimen for patients with a short treatment course of just four weeks," said Dr Mihaly Makara from the Buda Hepatology Centre, Budapest, Hungary, and lead study author. "We very much hope the long-term, 48-week follow-up data follows the same trend." This international study enrolled 79 patients with chronic HCV, genotype 1 or 4 who had not previously received treatment. Each patient received a 2mg/kg injection of RG-101 on Day one, with a four week course of oral DAAs (either ledipasvir/sofosbuvir, simeprevir, or daclatasvir), following by a second 2mg/kg injection of RG-101 on Day 29. The mean baseline viral load among patients was 5.805 IU/mL.
Interim analysis showed that 97.4% (37/38) and 100% (14/14) of patients at eight and 12 weeks respectiviely had a high virologic response. This was determined by assessing HCV levels 'below the lower limit of quantification' (<12 IU/mL), using the Abbot RealTimeHCV Assay, a consolidated HCV viral load and HCV genotype testing method.
The combination therapy was generally well tolerated, with the majority of side effects reported being mild in nature, including headache and fatigue, reported in 11.4% of patients.
"It is encouraging to see a potential treatment combination on the horizon that could limit treatment duration for patients," said Professor Tom Hemming Karlsen, EASL Vice Secretary. "We will be eagerly awaiting the 48 week follow-up data."
Advertisement
Source-Eurekalert