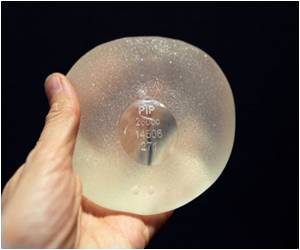
"Previous studies have typically reported failure rates of between two percent and five percent," said authors Miles Berry and Jan Stanek of the study published in the Journal of Plastic, Reconstructive and Aesthetic Surgery.
The new study had employed more conclusive ultrasound scans rather than the physical examinations typically used, they said.
"All PIP implants, due to the high rupture rate and uncertainty about the nature of the silicone gel, may need to be removed," Stanek was quoted as saying in a statement, adding that further research must be done.
"Being only a single-handed practice, this initial study is the tip of an iceberg that may affect 40,000 women in the UK with PIP implants," added a statement.
More than 400,000 women around the world are believed to have received implants made by French company PIP, which shut in 2010 after it was found to have used substandard, industrial-grade silicone gel.
Advertisement
France, Germany, the Czech Republic and the Netherlands have recommended the devices be removed as a precaution.
Advertisement
More than a dozen countries in Europe and Latin America have urged women to seek regular checkups.
Manufacturers and plastic surgeons say that breast implants have an expected, normal lifespan of about 12 years.
Usually in the case of rupture the silicone gel does not leak, but even it if does should not cause tissue irritation. However, in the case of the PIP implants, the gel used was of a non-medical grade.
"Those patients with ruptured implants will have to have them removed; those with no evidence of rupture will need to be monitored on a regular basis," said Stanek.
"Further research into the nature of the elastomer (outer casing) and gel filler will determine whether all PIP implants should be explanted in the future."
Source-AFP