"The results of the study suggest that use of low bone density medications may have a protective effect on endometrial cancer, or that women who take them get a less-aggressive cancer," says Sharon Hensley Alford, Ph.D, lead author of the study, and a researcher in Public Health Services at Henry Ford Hospital.
The classification for these medications is bisphosphonates.
In the fifth year of the ongoing trial, all participants were asked to complete a supplemental questionnaire, which included questions about their use of medications that treat thinning bones.
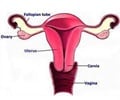
A total of 29,254 women were included in the analysis, for which 115 endometrial cancers have been diagnosed since the completion of the questionnaires.
The rate of endometrial cancer among women who had taken bisphosphonates was approximately half that of women who had never taken the medication (9.6 vs. 18.7 per 10,000 person years). The effect was more significant with less-aggressive cancers.
Advertisement
Henry Ford researchers used data from the National Cancer Institute's Prostate, Lung, Colorectal, and Ovarian Screening Trial, which collected data on all cancer outcomes for trial participants.
Advertisement
Women with missing information on bone medication use were excluded. Only women who had not had a hysterectomy were included in the analysis.
Women without a cancer diagnosis at the time of the questionnaire were separated into groups: those who reported current or past use of a bisphosphonate, defined as "ever used", and women who had never used such medications.
This study alone would not change clinical practice, and more study is necessary, according to Dr. Alford.
"This was a retrospective study, with self-reported data," says Dr. Alford. "A clinical trial, with closely monitored data, needs to be done for definitive results."
Source-Eurekalert