‘Patients in the orteronel group had significantly improved median progression-free survival (47.6 months versus 23.0 months) and prostate-specific antigen (PSA) response rates measured at 7 months after the start of treatment (complete-, partial-, and no-response rates of 58%, 22%, and 19% versus 44%, 31%, and 25%); these were secondary endpoints in the trial.’
Tweet it Now
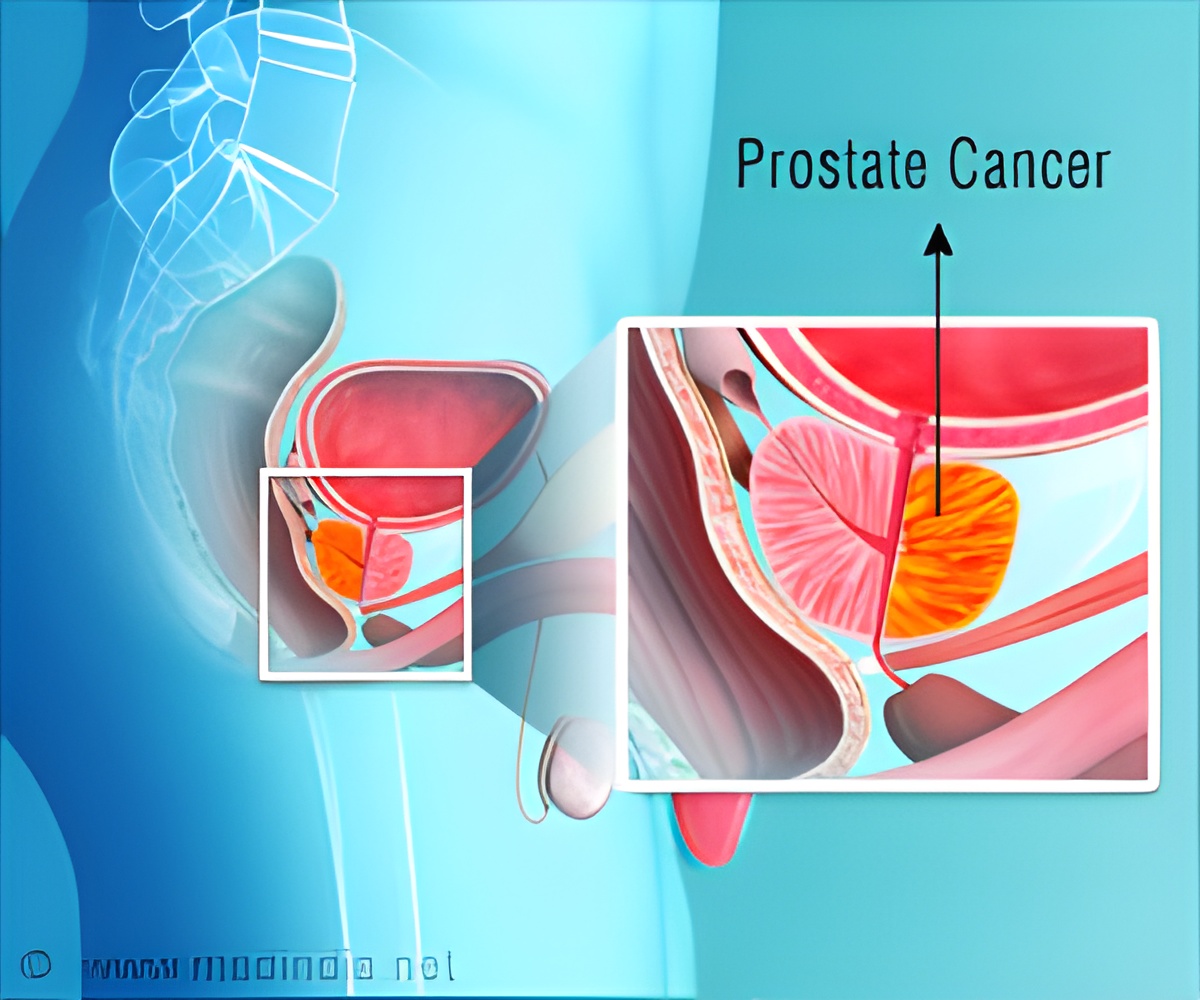
Although the study missed the primary endpoint of a 33% improvement in overall survival (OS), it also showed an unprecedented median OS of 70 months in the control group, the highest ever reported for these patients on a non-intensified androgen deprivation therapy group. This OS is a 24 month improvement over results reported in 2013 from the SWOG-9346 trial, which enrolled a nearly identical proportion of patients with extensive disease. Metastatic Prostate Cancer: New Findings
The researchers conclude that the primary reason for this extended survival, compared with previous studies, is the life-prolonging additional treatments patients received after they completed the S1216 trial. Some 77% of control patients whose cancer progressed went on to get additional life-prolonging treatment after finishing the trial therapy, compared with 61% in the orteronel group.“We are seeing the benefit of the advancements made in the advanced prostate cancer therapy in the last decade, resulting in unprecedented improvements in survival of men with advanced prostate cancer in general, which is great news for our patients,” said Neeraj Agarwal, a SWOG investigator with the Huntsman Cancer Institute at the University of Utah.
In setting the measure of success for the trial as an improvement in OS of at least 33%, the S1216 study team had worked from the assumption that the median control OS would be 54 months, a figure that built on the SWOG-9346 results and added time to account for the anticipated impact of new drugs then being reviewed for approval. Because the actual median control OS exceeded this assumption by 16 months, the results did not meet the threshold for S1216 to be considered a positive trial.
Source-Eurekalert







![Prostate Specific Antigen [PSA] Prostate Specific Antigen [PSA]](https://www.medindia.net/images/common/patientinfo/120_100/prostate-specific-antigen.jpg)