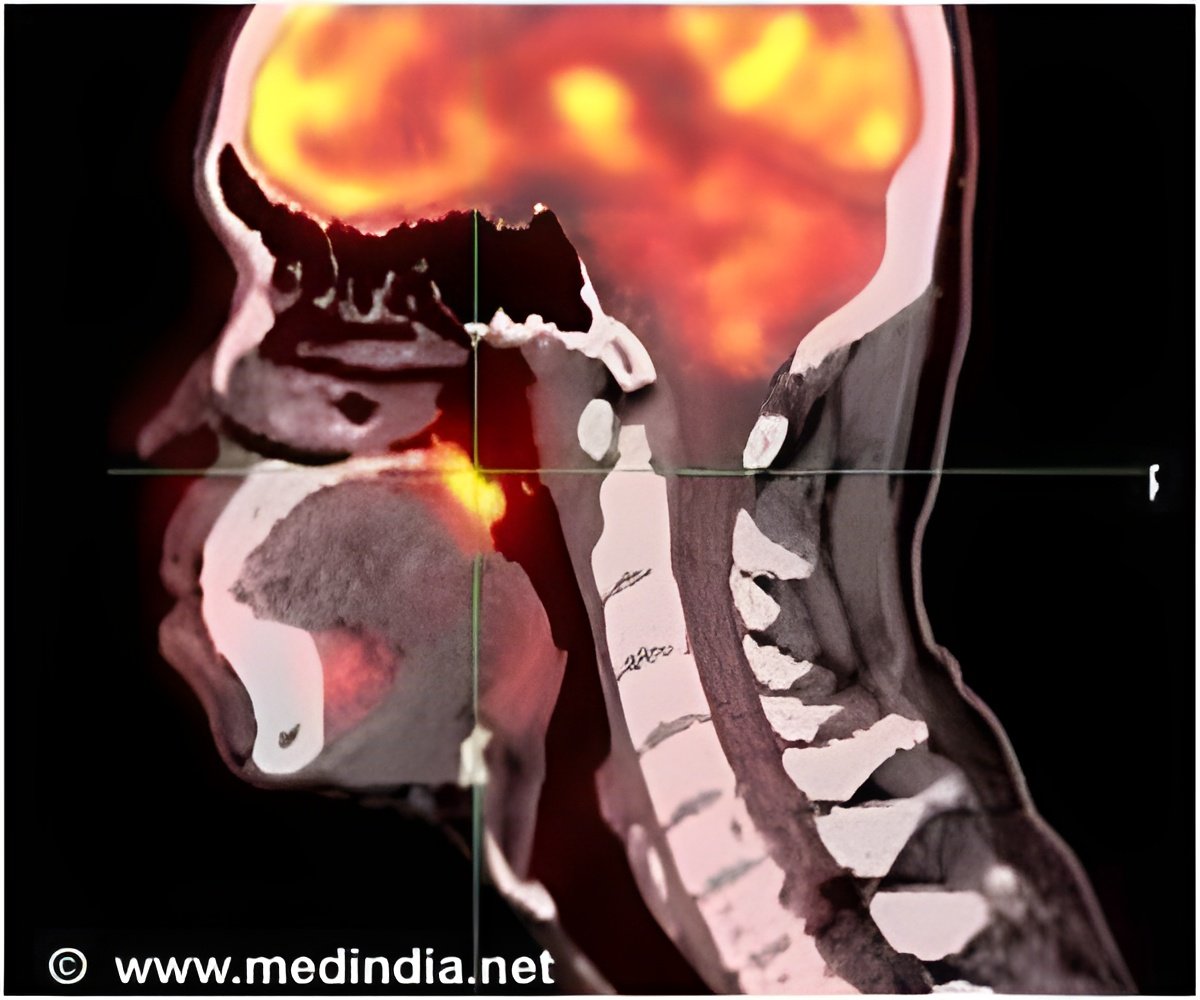
Prior research has established that HPV is a cause of some head and neck cancers, including oropharyngeal cancer, and that patients with HPV-associated disease tend to have a better clinical outcome. Consequently, the proper assessment of the clinical status of individual tumors has become a goal of clinicians treating this disease because HPV at the tumor site does not indicate causal involvement in the cancer.
In the first study, Dana Holzinger, Ph.D., of the division of genome modifications and carcinogenesis at the German Cancer Research Center in Heidelberg, Germany, and colleagues analyzed the potential of direct and indirect HPV markers to identify patients with HPV-driven tumors.
They analyzed 199 oropharyngeal squamous cell carcinoma specimens for HPV DNA, viral load, RNA expression patterns seen in cervical carcinomas and the p16 protein, which is associated with tumor suppression.
Results indicated that the cervical cancer RNA expression pattern and viral load were associated with the lowest risk for death from oropharyngeal cancer. In contrast, a weaker association was found for samples that were HPV DNA-positive or that expressed the p16 protein.
"We showed that high viral load and a cancer-specific pattern of viral gene expression are most suited to identify patients with HPV-driven tumors among patients with oropharyngeal cancer," Holzinger said. "Viral expression pattern is a completely new marker in this field and viral load has hardly been analyzed before."
Advertisement
They found that the expression of two oncoproteins, E6 and E7, was associated with improved survival in oropharyngeal disease. In addition, HPV DNA positivity or p16 expression combined with E6 and E7 expression were also associated with enhanced survival. However, neither HPV DNA positivity nor expression of p16 alone yielded a similar result.
Advertisement
The next step in this research is further validating the findings of these two studies using head-to-head comparisons and developing assays for direct clinical application of the markers.
"Once standardized assays for these markers, applicable in routine clinical laboratories, are established, they will allow precise identification of patients with oropharyngeal cancer with or without HPV-driven cancers and, thus, will influence prognosis and potentially treatment decisions," Holzinger said.
# # #
Follow the AACR on Twitter: @aacr #aacr
Follow the AACR on Facebook: http://www.facebook.com/aacr.org
About the AACR
Founded in 1907, the American Association for Cancer Research (AACR) is the world''s first and largest professional organization dedicated to advancing cancer research and its mission to prevent and cure cancer. AACR''s membership includes 34,000 laboratory, translational and clinical researchers; population scientists; other health care professionals; and cancer advocates residing in more than 90 countries. The AACR marshals the full spectrum of expertise of the cancer community to accelerate progress in the prevention, biology, diagnosis and treatment of cancer by annually convening more than 20 conferences and educational workshops, the largest of which is the AACR Annual Meeting with more than 17,000 attendees. In addition, the AACR publishes seven peer-reviewed scientific journals and a magazine for cancer survivors, patients and their caregivers. The AACR funds meritorious research directly as well as in cooperation with numerous cancer organizations. As the Scientific Partner of Stand Up To Cancer, the AACR provides expert peer review, grants administration and scientific oversight of individual and team science grants in cancer research that have the potential for near-term patient benefit. The AACR actively communicates with legislators and policymakers about the value of cancer research and related biomedical science in saving lives from cancer.
For more information about the AACR, visit www.AACR.org.
Source-Newswise