‘CAR T cells are a form of immunotherapy involving the engineering of T cells, a type of immune cell that fights cancer.
’
Tweet it Now
The researchers report that this approach was effective in treating these cancers in mouse models of human disease. The findings support further clinical studies to evaluate this strategy to treat pediatric brain cancers, the most common cause of cancer death in childhood. Indeed, a first-in-child clinical trial currently is recruiting patients at Texas Children's Hospital and Baylor College of Medicine to test the safety and anti-tumor efficacy of this approach (Clinicaltrials.gov identifier: NCT02442297)."Recurrences of medulloblastoma and ependymoma can be disseminated throughout the lining of the brain and spinal cord, which are bathed in cerebrospinal fluid. This location offers the opportunity to deliver therapies into the cerebrospinal fluid compartment and could provide a better chance for the therapy to reach and eliminate the tumor than administering it through the bloodstream," said co-corresponding author Dr. Nabil Ahmed, associate professor of pediatrics and immunology, a section of hematology-oncology at Baylor and Texas Children's Hospital.
"The vast majority of children with recurrent metastatic medulloblastoma or ependymoma currently have a deadly prognosis, so it is very exciting to think we have identified a novel approach to treat this underserved patient population," said co-corresponding author Dr. Michael Taylor, neurosurgeon, a senior scientist in the Developmental and Stem Cell Biology program, Garron Family Chair in Cancer Research at SickKids, and professor in the Departments of Surgery and Laboratory Medicine and Pathobiology at the University of Toronto.
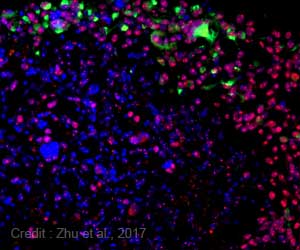
This project was led by Dr. Laura Donovan, a post-doctoral fellow in the Developmental and Stem Cell Biology program at SickKids, who performed in-depth molecular studies of the target profile of recurrent medulloblastoma and ependymoma. These studied guided the design of CAR T cells engineered by Ahmed and colleagues at Baylor's Center for Cell and Gene Therapy and Texas Children's Hospital to target the most appropriate cancer molecules.
The researchers genetically engineered CAR T cells to recognize specific molecules on the surface of the tumor cells. When these CAR T cells encounter the tumor, they can fight it more effectively. CAR T cells have been impressively effective for patients with certain types of leukemia and are FDA-approved for this disease.
Advertisement
The results showed that administering tumor-specific CAR T cells into the cerebrospinal fluid was more effective than administering them via the blood.
Advertisement
In some of their experiments, the researchers combined CAR T cells with an approved cancer medication called azacytidine. The results showed that combining immunotherapy with azacytidine was significantly more effective than either treatment alone.
"This work was possible thanks to the concerted collaboration of our Pediatric Cancer Dream Team supported by Stand Up To Cancer® (SU2C) St. Baldrick's Foundation Translational Research Grant, which brought together scientists studying tumor genomics and tumor immunotherapy around the world to enable the design of more effective therapies for children with incurable and hard to treat cancers," Ahmed said.
Source-Eurekalert