The Phase I trial will enroll up to 30 healthy men and women ages 18 to 45 in a dose-escalation study to evaluate the investigational drug’s safety and tolerability. Quintiles, through Dynport Vaccine Company, is conducting the study of CRS3123. The medication is being provided by its developer, Crestone, Inc., a pharmaceutical company based in Boulder, Colo.
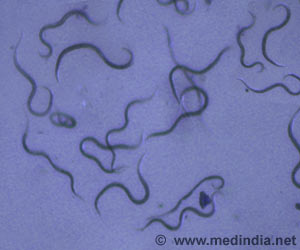
C. difficile infection affects the digestive track with manifestations ranging from mild diarrhea to life-threatening colitis. Infection most commonly affects patients in hospitals or long-term care facilities and people who have taken antibiotics. Each year, roughly 250,000 people in the United States require hospital care for C. difficile infection and at least 14,000 people die from the infection, according to the U.S. Centers for Disease Control and Prevention (CDC). As a result, the CDC identified C. difficile as an urgent public health threat in its 2013 report on antibiotic resistance. Resistance to the antibiotics currently used to treat C. difficile is not yet a problem, but the bacteria spreads rapidly because it is naturally resistant to many drugs used to treat other infections. Recurrent infections pose a particular clinical challenge, especially those caused by a newer, stronger C. difficile strain, which result in up to 25 percent of patients needing additional therapy.
In the new clinical trial, participants will be randomly assigned into three groups of 10 participants each (8 participants will receive the investigational drug; 2 participants will receive a placebo pill). In the first group, participants will receive either 200 milligrams (mg) of CRS3123 or placebo twice daily for 10 days. Based on the review of safety data from this group, the study will progress to a second and then a third group of volunteers with a maximum possible dose of 600 mg twice daily. Participation in the trial will require an inpatient stay of 12 days to allow for monitored dosing and physical evaluations, including blood tests and urinalysis. Participants will be evaluated on an outpatient basis 18 and 29 days after receiving the first dose of the investigational drug. The study is expected to be completed by March 2015.
Source-Eurekalert