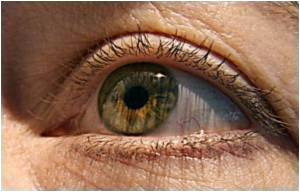
Leading drug maker Novartis received a major blow on Friday after the British health agency revealed that it has rejected the approval for Lucentis for treating Diabetic Macular Oedema (DMO).
Lucentis has been in news for some time due to its heavy price tag with the drug costing over $1,200 for each injection. The drug was discovered by Roche’s US biotech firm, Genentech, and has proved to be successful for both Novartis and Roche, with annual sales exceeding $1.5 billion.
Though Lucentis is already available in the British market, with the National Institute for Health and Clinical Excellence (NICE) approving it for wet age-related macular degeneration, the health agency felt that the drug did not provide value for money when treating DMO.
Instead Roche’s cancer drug, Avastin, has proven to be as effective in treating DMO and is available at a fraction of Lucentis’ price at $50 per injection.
Source-Medindia