Scientists have developed new Non-Invasive Ultrasound Therapy (NIUT) for the treatment of cardio-valvular diseases such as aortic stenosis.
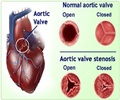
‘Aortic stenosis is the primary valve disease in adults and one of the leading causes of cardiovascular death worldwide which affects 10 million people in Europe and the USA.’





The mechanism of this groundbreaking technology developed by Cardiawave consists in widening the opening of the aortic valve by delivering short sequences of high intensity ultrasounds, focused directly onto the valve from outside the body, thus eliminating the need for invasive treatment. The results of the study are very promising. Analysis of the patients' heart condition by echocardiography performed by an independent core laboratory, and of the clinical results at 6 months, confirms that this procedure is safe and can improve the condition of the heart and the clinical status of these very sick patients. For them, this new therapy can be a major therapeutic opportunity given the absence of other solutions.
In the near future, this therapy might be used for other indications, such as to improve patients' condition and prepare them for TAVR (bridge to TAVR) and for less sick patients (severe asymptomatic and moderate aortic stenosis) to slow down the progression of their disease and delay the moment they need replacement of their valves.
This therapy can be done ambulatory and can be very cost-effective. In addition, as it is non-invasive, it most probably can be repeated to further improve or maintain its effect.
This work is supported by the French Government, managed by the National Research Agency (ANR) under the program "Investissements d'Avenir" with the references ANR-16-RHUS-0003_STOP-AS and ANR-17-CE 19-00 19-03 and has received funding from the European Union's Horizon 2020 research and innovation programme under accreditation n° 829492.
Advertisement