‘New drug compound MK-6482 is a well-tolerated medication with a favorable safety profile. It holds promise in treating kidney tumor patients.’
Read More..Tweet it Now
"A new drug as a single agent showing an overall response rate of 24 percent across all risk categories - poor, intermediate, and good and in a heavily refractory population - is quite promising," said Toni Choueiri, MD, first author of the abstract. Choueiri is director of the Lank Center for Genitourinary Oncology and the Jerome and Nancy Kohlberg Professor of Medicine at Harvard Medical School.Read More..
The drug targets a component of the body’s mechanism for sensing oxygen levels and turning on genes that enable the body to adjust to hypoxia - a shortage of oxygen - by making more red blood cells and forming new blood vessels.
Dana-Farber scientist and Choueiri’s mentor and collaborator William G. Kaelin Jr., MD shared the 2019 Nobel Prize in Medicine with two other researchers for unraveling this complex mechanism, which can be hijacked by cancer to help tumors survive and grow.
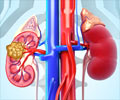
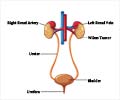
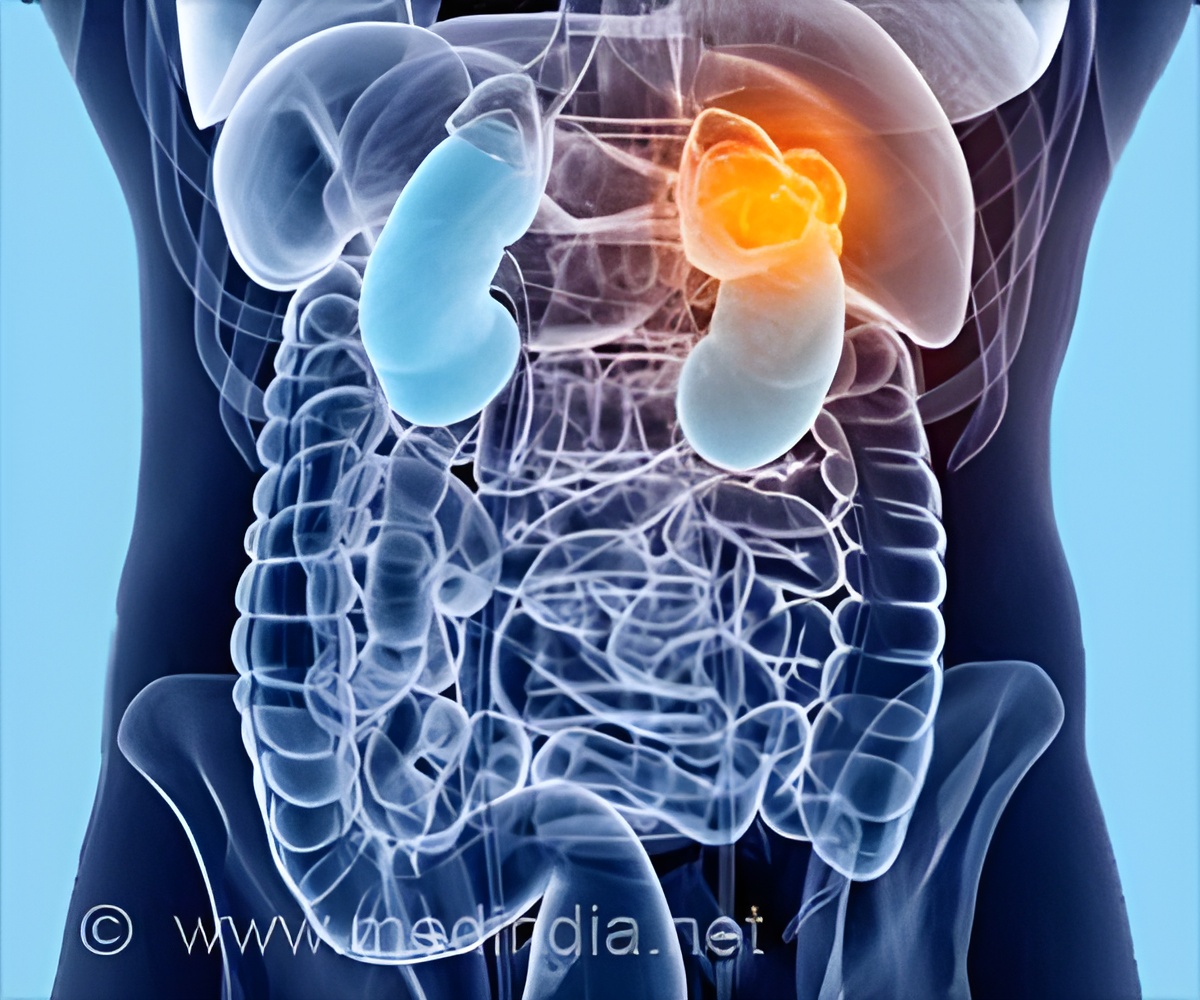
In the vast majority of patients with clear cell renal carcinoma, a tumor suppressor protein known as Von Hippel-Lindau (VHL) is not functional. As a result, hypoxia inducible factor (HIF) proteins accumulate inside the tumor cell, wrongly signaling there is a shortage of oxygen, and activating the formation of blood vessels, fueling tumor growth.
Understanding this abnormal process has paved the way for new cancer drugs - MK-6482 being one of them and is distinct in that it targets HIF-2a directly leading to blocking cancer cell growth, proliferation, and abnormal blood vessel formation.
Advertisement
After a median follow-up period of 13 months, the overall response rate was 24 percent. Forty-one patients had stable disease with a disease control rate (complete response plus partial response plus stable disease) of 80 percent. There were partial responses in two of five favorable-risk patients; 10 of 40 intermediate-risk patients; and one of 10 poor-risk patients.
Advertisement
The authors concluded that MK-6482 "is well-tolerated with a favorable safety profile and demonstrated promising single-agent activity in heavily pre-treated patients" with clear-cell kidney cancer across the various risk groups.
Source-Eurekalert