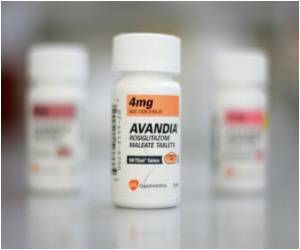
The document cited Thomas Marciniak, an FDA expert on cardiovascular treatments, who indicated that clinical trials of the drug, whose generic name is rosiglitazone, were "inadequately designed" and that the record "suggests the rosiglitazone increases the risk" of heart attacks.
"Half of the errors were substantial," Marciniak said in the document. "If consulted in advance, I would have rejected this study design as inappropriate and biased."
The report came after two studies released in recent weeks concluded patients taking Avandia faced a higher risk of heart attacks and strokes, emboldening critics who have asked that it be withdrawn from the market.
The FDA often follows the advice of its panels of experts, which will make its recommendation after the talks on Tuesday and Wednesday.
In addition to Marciniak's comments, the FDA also released some 700 pages of documents examining studies on Avandia and its safety compared with other treatments.
Advertisement
Source-AFP