‘Tau proteins modulate the stability of axonal microtubules and are found abundantly in neurons of the central nervous system.’
Tweet it Now
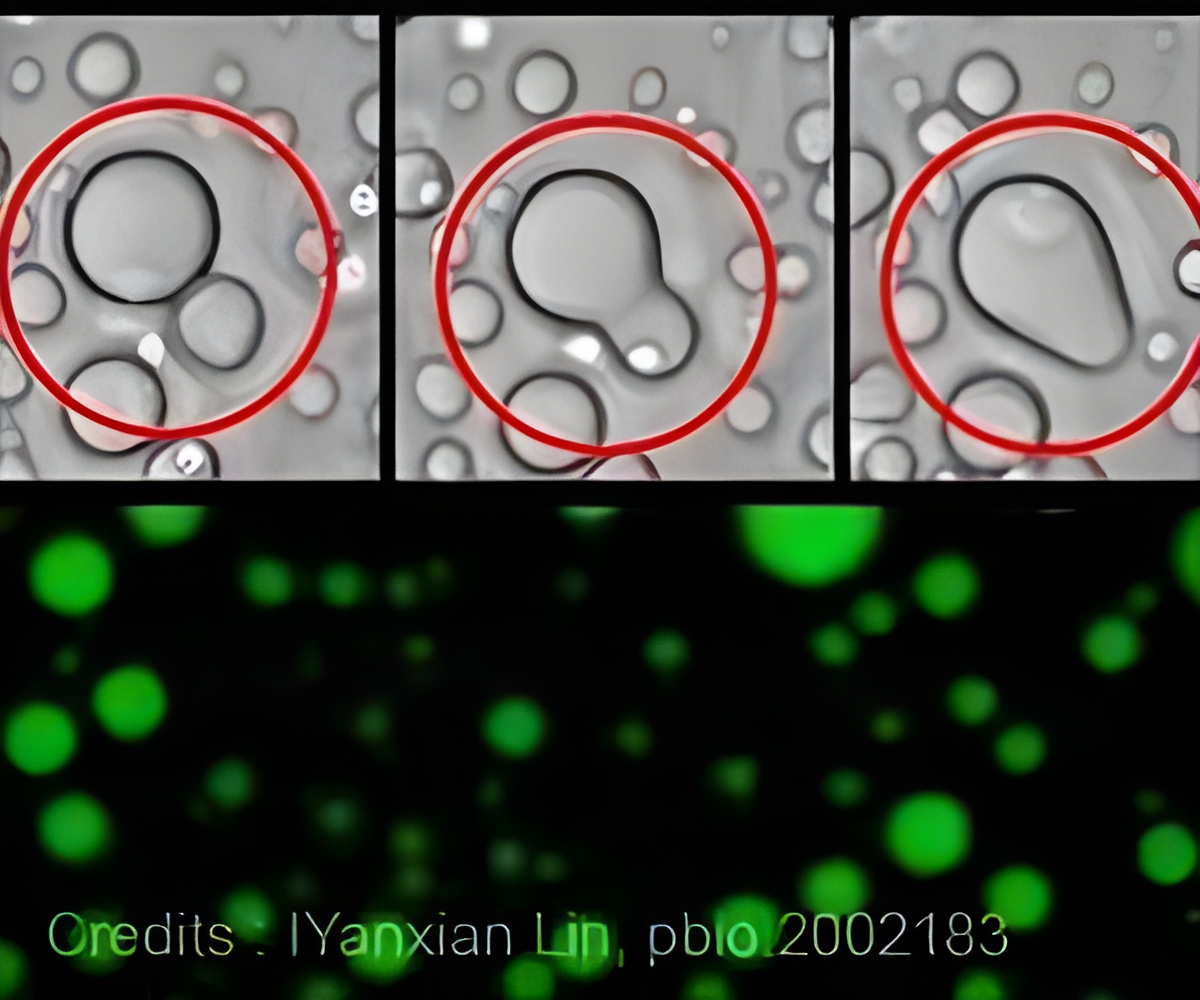
The authors show that tau protein can condense into a highly compact "droplet" known as a complex coacervates in a complex with RNA while retaining its liquid properties. Tau and RNA are held together in phase separation from the surrounding environment, and despite the high concentration, the tau protein initially avoids assembling into fibers. However, this novel state creates a set of conditions in which tau becomes vulnerable to aggregation.
Depending on the length and shape of the RNA, increasing numbers of tau molecules can loosely associate with the complex. These properties have been observed with several other neurodegenerative disease-related proteins, such as those that cause amyotrophic lateral sclerosis.
The findings provide a biophysical clue on the path to understanding tau pathology. Critical steps along this path include the intrinsic ability of tau to fold into a myriad of shapes, its ability to bind to RNA, and its ability to form compact reversible structures under physiologic conditions.
Future research is required to identify the counterpart of tau droplets in living cells, and to determine how and why a cell regulates the formation and dissolution of these droplets, insights that could lead to potential therapies.
Advertisement
Source-Eurekalert