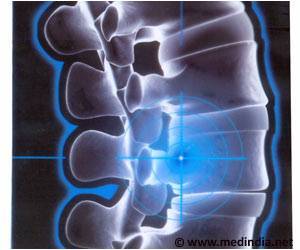
‘Nusinersen drug is the first FDA approved therapy for spinal muscular atrophy and is administered into the cerebrospinal fluid by repeat lumbar puncture every 4 months for life. The new drug delivery method makes it simpler with a subcutaneous intrathecal catheter system (SIC) that replaces the painful repeated lumbar puncture.’
Tweet it Now
Nusinersen is the first FDA approved therapy for SMA but must be administered into the cerebrospinal fluid by repeat lumbar puncture every 4 months for life. It is done using a subcutaneous intrathecal catheter system (SIC) .Spinal muscular atrophy (SMA) is the leading genetic cause of infant death worldwide. Unfortunately, the majority of surviving SMA patients have skeletal deformities or spinal hardware that make it difficult to safely and reliably access the cerebrospinal fluid.
The study, by clinicians and researchers at the Clinic for Special Children in Strasburg, PA and the Nemours/A.I. DuPont Hospital for Children in Wilmington DE, appears in the Journal of Pediatric Orthopaedics. Ten SMA patients underwent implantation of the catheter device and received nusinersen dosing through the SIC.
The device implantation took less than two hours and was well tolerated in all patients, with an average hospital stay of less than 55 hours.
Once the SIC system was implanted, all subsequent nusinersen doses were administered in less than 20 minutes, requiring only topical anesthetic in an outpatient setting.
Advertisement
This SIC dosing method might also benefit SMA patients without advanced neuromuscular disease or spinal pathology.
Advertisement
While the study's observations suggest the SIC system to be relatively safe and without complications, use of the device warrants further multicenter trials.
If proven effective in future studies, this method could double the number of patients able to receive nusinersen worldwide and reduce costs five- to ten-fold.
Source-Eurekalert