The drug has been accredited for once daily use and can be utilized over a long period of time.
“The availability of this new long-term maintenance medication offers an additional treatment choices for the millions of people who suffer from COPD,” noted Curtis Rosebraugh, MD, MPH, director of the Office of Drug Evaluation II in the FDA’s Center for Drug Evaluation and Research in the statement.
As a long-acting beta adrenergic agonist (LABA) Striverdi Respimat is designed to help keep muscles around a patient’s airways relaxed in order to help manage their symptoms.
The drug was examined in a study that included 1304 patients with those who were prescribed the drug showing more improvement than those taking a placebo.
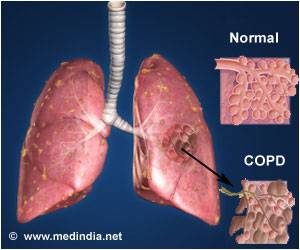
Symptoms of COPD can comprise wheezing, coughing, chest tightness and shortness of breath.
According to the National Heart Lung and Blood Institute, it is the third leading cause of death in the US.
Advertisement
The FDA stated, the safety and effectiveness for using the drug for the treatment of asthma has not been established and it is not currently an officially accepted use. It also should not be used as a rescue therapy.
Advertisement
The drug is also not advised for patients with acutely deteriorating COPD as it may produce serious side effects including narrowing and obstruction of the respiratory airway (paradoxical bronchospasm) and other cardiovascular effects.
Side effects associated with the drug include runny nose, upper respiratory tract infections, bronchitis, cough, urinary tract infection, dizziness, rash, diarrhea, back pain and joint pain.
The drug is supplied by Boehringer Ingelheim Pharmaceuticals, Inc. of Ridgefield, CT.
Source-Medindia

![Pulmonary Arterial Hypertension [PAH] - Symptoms & Signs - Causes - Diagnosis - Treatment Pulmonary Arterial Hypertension [PAH] - Symptoms & Signs - Causes - Diagnosis - Treatment](https://www.medindia.net/images/common/patientinfo/120_100/pulmonary-arterial-hypertension-pah.jpg)