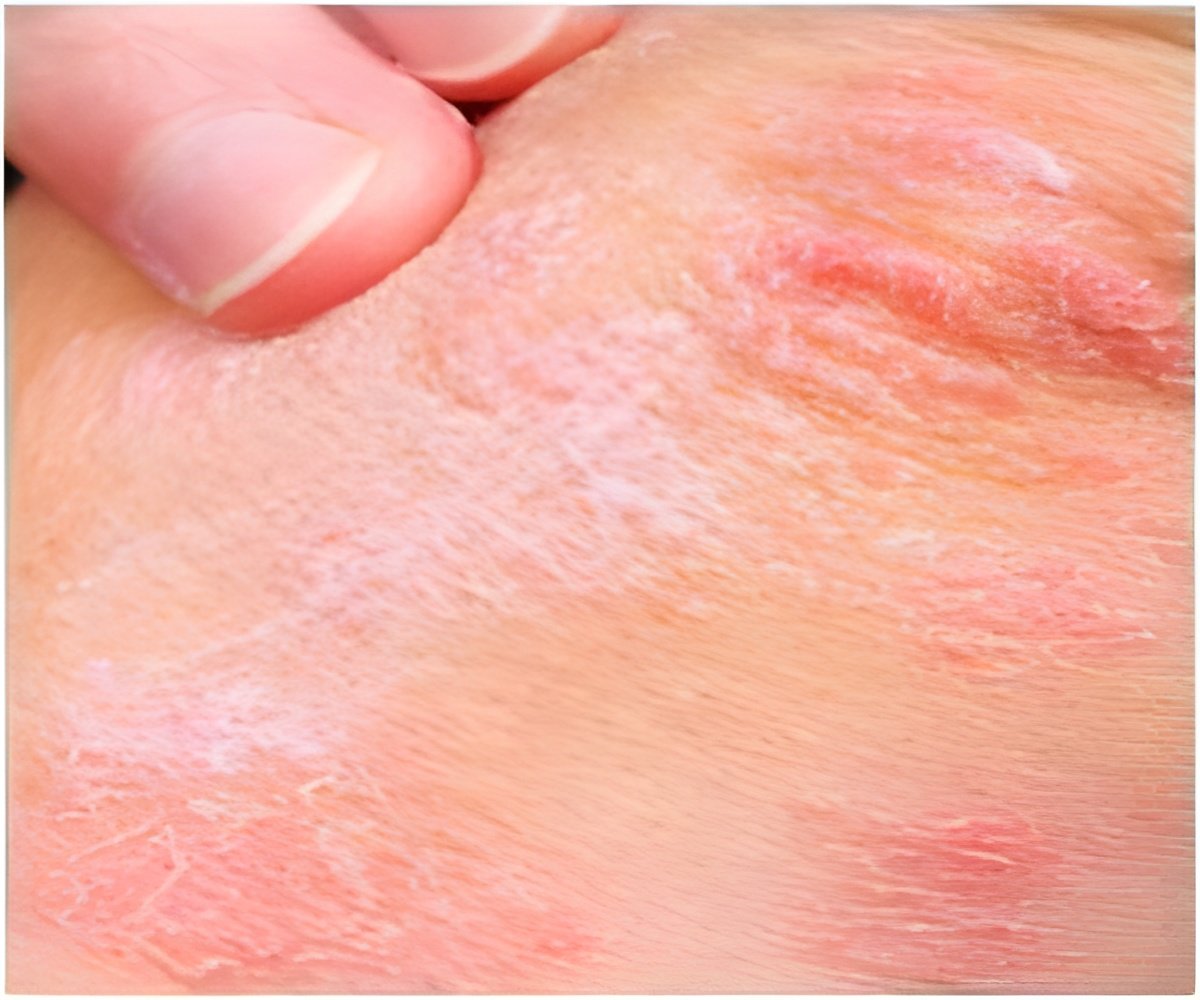
Moderate-to-severe AD is a more chronic version of the disease that typically has more systemic features and is seen in up to 3 percent of adults with the disease. AD can impact a person's ability to lead a full and active life. In addition, people with AD are more likely to have asthma and other allergic disorders such as hay fever. Current treatments for AD include topical and oral steroids as well as phototherapy, but their effectiveness is limited or the side effects associated with their chronic use are significant.
"We are encouraged by the consistent findings across these studies, which show that patients treated with dupilumab had a marked improvement in disease activity and itch," Beck said. "At this point, dupilumab appears to be remarkably effective for adults with severe AD, although larger studies are needed to confirm its safety and efficacy."
Dupilumab is administered as a skin injection, and has shown promise in both Phase I and Phase II studies. Participants in a 12-week Phase II study showed a 74 percent reduction in the Eczema Area Severity Index, a tool used to measure the severity of a patient's condition, compared to only 23 percent in the placebo group. The majority of patients in the group receiving dupilumab experienced significant reductions in itch.
The study's findings set the stage for Phase III clinical trials of dupilumab, to confirm its effectiveness, monitor side effects, and compare it to commonly used treatments.
Dupilumab is an investigational monoclonal antibody developed by Regeneron Pharmaceuticals, Inc., and Sanofi.
Advertisement
Source-Eurekalert