"At this point in FDA's investigation, the sterility of any injectable drugs.. and cardioplegic solutions" produced by the firm are "of significant concern," the FDA said in a statement.
The New England Compounding Center (NECC) recalled all of its products on October 6 after dozens of people were infected with meningitis following treatment with its steroid injections.
Health officials in 23 states sought out the nearly 14,000 people who received the potentially tainted doses in recent months to warn them of the danger.
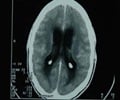
They have identified 205 cases of meningitis -- and 15 deaths -- so far and the numbers have been rising daily.
A long incubation period has complicated efforts -- one patient did not develop symptoms until 42 days after receiving a tainted injection.
Advertisement
Two transplant patients who received a solution produced by NECC to induce cardiac muscle paralysis during open heart surgery have subsequently developed the rare fungal meningitis, the FDA said.
Advertisement
The FDA cautioned that it has "not confirmed that these three infections were, in fact, caused by an NECC product."
"Investigation of these patients is ongoing; and there may be other explanations for their Aspergillus infection," the FDA said in a statement.
The outbreak has led to calls for tighter regulation of the loosely controlled pharmaceutical compounding industry.
Critics said drug manufacturers have found a way to sidestep costly and strict oversight by classifying themselves as pharmacies, which are given freer rein to mix drug compounds for patients.
Source-AFP