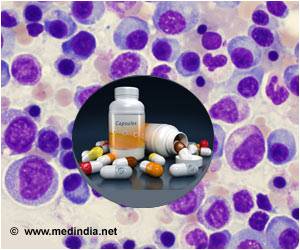
‘Multiple Myeloma is a type of a blood cancer affecting the infection-fighting region of plasma cells which then multiply and produce abnormal proteins.’
Tweet it Now
Study participants received either Ninlaro in combination with lenalidomide and dexamethasone or placebo plus lenalidomide and dexamethasone. The results showed that participants taking the once-weekly regimen lived for an average of 20.6 months compared to 14.7 months in the group receiving a placebo plus Revlimid and dexamethasone, with a tolerable safety profile.”The introduction of Ninlaro marks an important step forward, as its efficacy and safety profile - coupled with its completely oral administration - potentially can reduce some logistical burdens, and help enable patients to reap the full benefits of this sustainable therapy,” noted Christophe Bianchi, president of Takeda Oncology.
Source-Medindia