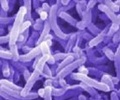
Opisthorchiasis is caused by a fluke, known by its Latin name as Opisthorchis viverrini, that passes from freshwater snails to river fish, and then to humans if the fish is eaten raw or undercooked.
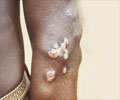
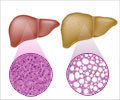
The fluke holes up in the liver and bile duct, where it reproduces, causing abdominal pain and diarrhoea but also, in the long term, boosting the risk of jaundice, gallstones and cancer.
Around 67 million people in Southeast Asia are at risk from the fluke, and nine million people have already been infected by it, according to a 2005 estimate.
The parasite is endemic to parts of Cambodia, Thailand and Vietnam, but especially in Laos, where as many as 50 percent of schoolchildren and 90 percent of adults are infected.
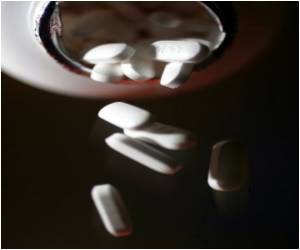
A team led by Jennifer Keiser from the Swiss Tropical and Public Health Institute randomly assigned 125 infected schoolchildren in Laos' Attepeu province to receiving either tribendimidine, praziquantel or a drug designed to combat the parasite that causes malaria.
Advertisement
Both tribendimidine and praziquantel also scored best in clearing the patient of parasite eggs, and were generally well tolerated, with "mild or moderate" side-effects.
Advertisement
Source-AFP