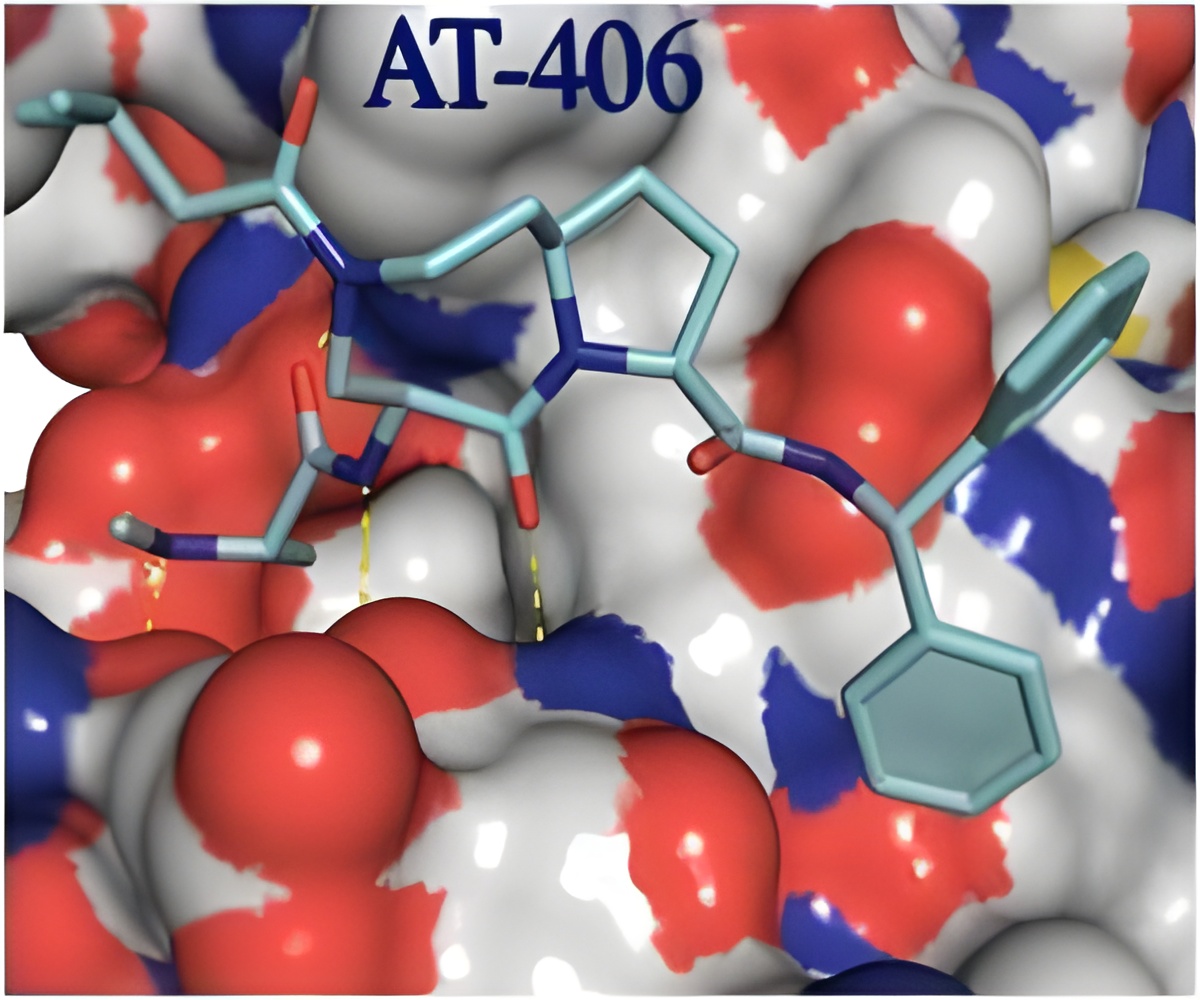
The normal cell death process, called apoptosis, is what keeps normal cells in check. When apoptosis is disrupted, cells reproduce uncontrollably, which is a hallmark of human cancer.
Advertisement
Wang's laboratory has been pursuing new cancer treatments aimed at this cell death pathway since 2003. His team designed and made AT-406 and tested it in the laboratory in 2006. The small-molecule drug hones in directly on the proteins called inhibitor of apoptosis proteins or IAPs that block cell death. The researchers found that AT-406 destroyed these proteins in cancer cells. Meanwhile, the drug had little to no effect on normal cells.
In animal models, the drug shrank tumors but caused few side effects. The drug is designed to be taken by mouth, which researchers say will make it easier than traditional intravenous chemotherapies to administer.
Patent applications covering the drug are exclusively licensed to Ascenta Therapeutics, a privately-held, clinical stage biopharmaceutical company co-founded by Wang. After extensive testing, Ascenta began the first clinical trial in 2010 testing AT-406 for cancer treatment. This trial, which is being tested in all solid tumors, is offered at the U-M Comprehensive Cancer Center, Duke University and the Mayo Clinic. Ascenta has also recently opened a second trial of AT-406 in high-risk acute myeloid leukemia at the U-M Comprehensive Cancer Center. Several more clinical trials are planned.
Advertisement
Source-Newswise