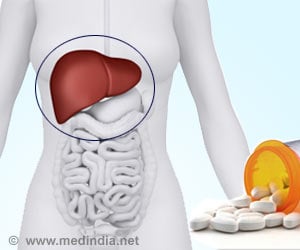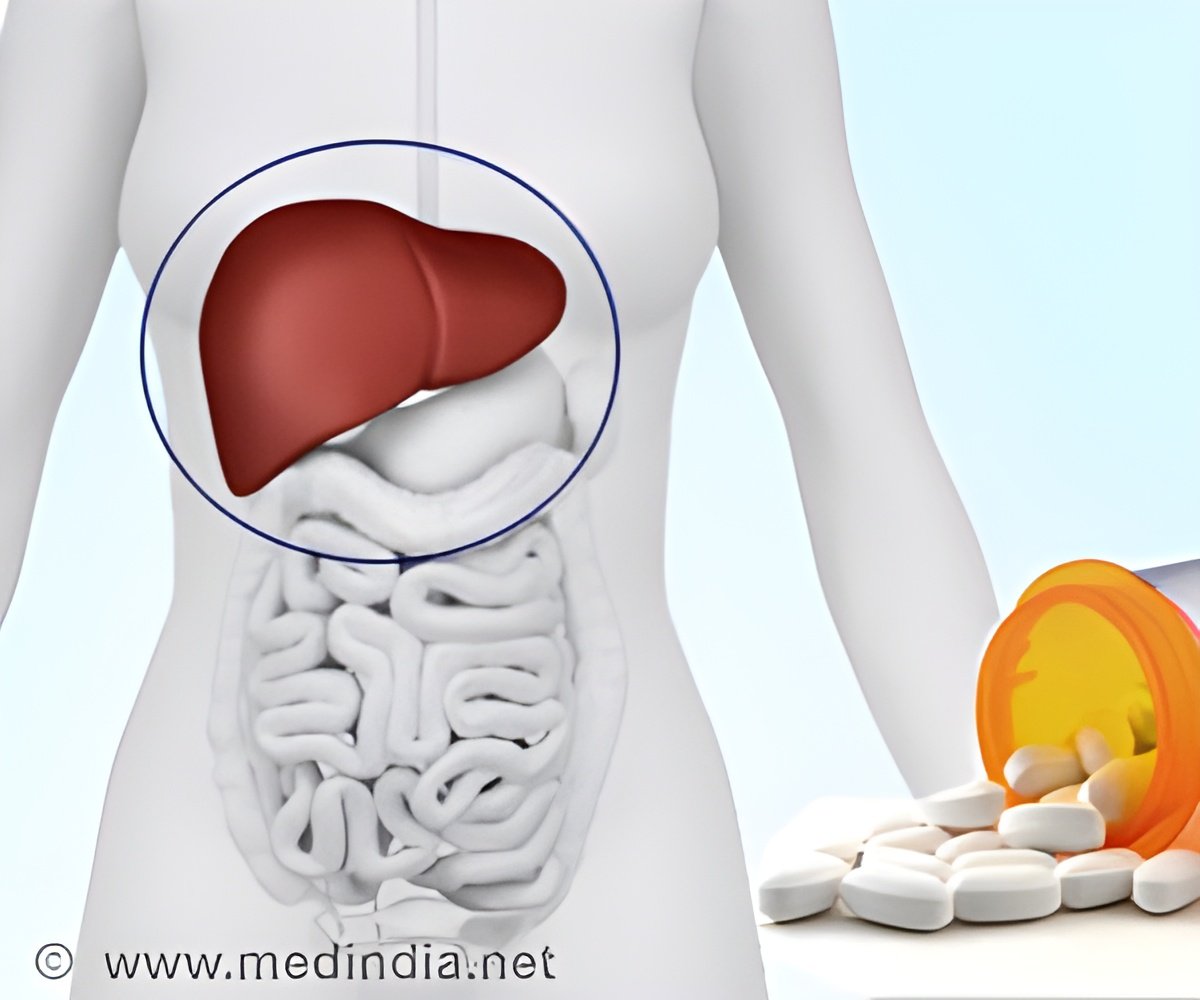
‘Drug Controller General of India approves the sale of generic version of Daclatasvir tablets in the strengths of 30 mg and 60 mg for the treatment of chronic hepatitis C virus infection.’
Tweet it Now
Natco Pharma will sell the drug under its brand name ‘NATDAC’ and has received approval for two dosage levels, 60mg and 30 mg. The price is Rs, 6000 for 60mg and Rs. 4000 for 30 mg, for a bottle of 28 tablets.“It is the first company in India to get approval for generic Daclatasvir Dihydrochloride (Daclatasvir) tablets, 30 mg & 60 mg, from the Drugs Controller General (India),” Natco said.
”Compared with other treatment options, this combination not only increases the cure rate, but is also regarded as a valuable treatment option in some of the difficult-to-treat HCV patients subsets,” Natco added.
Hetero said its product is generic version of Bristol-Myers Squibb's Daklinza tablets.
"The product in combination with Sofosbuvir is indicated for the treatment of Hepatitis C genotype 3 infections, which is highly prevalent in India," Hetero added.
Advertisement