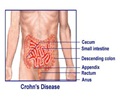
‘Following the protocol for biomarker and symptom monitoring improves mucosal healing, the complete absence of all ulcerative lesions in all segments of the gut after 48 weeks.’
Tweet it Now
In addition, a significantly higher proportion of patients in the TC group achieved deep remission and steroid-free remission compared with the CM group. The trial, known as CALM, "Open-Label, Multi Center, Efficacy and Safety Study to Evaluate Two Treatment Algorithms in Subjects with Moderate to Severe Crohn’s Disease" is the first study to demonstrate that monitoring inflammation biomarkers, including C-reactive protein (CRP) and fecal calprotectin, along with clinical symptoms led to superior patient outcomes while using anti-tumor necrosis factor (TNF) therapy (adalimumab/Humira) compared with symptom-driven decisions alone.
"With this study, we have implemented a new concept of ’tight control’ of Crohn’s disease, which will change the way that patients will be followed in clinical practice," said the paper’s lead author, Jean-Frederic Colombel, MD, Director of The Susan and Leonard Feinstein.
"Treatment should be based on objective markers of inflammation and not only on symptoms. A patient with Crohn’s disease may do well clinically, but if biomarkers for the disease remain high, we still need to escalate interventions including drugs. This study shows tight control led to more patients experiencing clinical remission, which will ultimately improve their long-term outcomes."
In the phase 3, multi-center clinical trial, 244 patients with moderate to severe Crohn’s disease were randomly assigned in equal numbers to TC or CM groups after eight weeks of prednisone induction therapy, or earlier if they had active disease.
Advertisement
In the TC group, drugs were escalated to the next step when CRP rose above 5mg/L or fecal calprotectin above250 ?g. In both groups, drugs were also escalated to the next step when patients reported increases in clinical symptoms.
Advertisement
"When patients with Crohn’s disease are making therapeutic decisions about starting a drug, or escalating or de-escalating a drug, they can’t base these decisions on symptoms alone," said Dr. Colombel. "They need objective data including biomarkers and endoscopy results. Ultimately this will disable their disease from progressing."
Source-Eurekalert