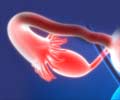
A molecule that can disrupt the gene responsible for causing Ewing's sarcoma has been identified by American scientists. Ewing's sarcoma is a rare cancer occurring in children and young adults
The breakthrough results form the efforts of scientists at Georgetown University Medical Center, who say that further studies may establish the novel agent as the first targeted therapy to treat Ewing's sarcoma, wherein tumours can occur anywhere in the body.Making a presentation at the annual meeting of the American Association for Cancer Research (AACR) in San Diego, the researchers revealed that the activity of the molecule was based on a protein-protein interaction.
They further said that their findings suggested that the same interaction could provide a model upon which other therapies could be designed.
"I think this holds really wonderful promise as a unique way of targeting fusion proteins. People thought it wasn't possible to have a small molecule that can bind between flexible proteins, but we have shown that it can be done," said lead researcher Dr. Jeffrey Toretsky, a pediatric oncology physician and researcher at Georgetown University's Lombardi Comprehensive Cancer Center.
Toretsky conceded that more research was required before the novel agent could be tested in humans, as laboratory cells were used for the study. He revealed that further lab studies were underway.
Ewing's sarcoma results from the exchange of DNA between two chromosomes, a process known as a translocation. The new gene, EWS-FLI1, is created when the EWS gene on chromosome 22 fuses to the FLI1 gene on chromosome 11, and its product is the fusion protein responsible for cancer formation.
Advertisement
"We did this in order to find out if EWS-FLI1 might be binding with other cellular proteins," he says.
Advertisement
"We believe that when RHA binds to EWS-FLI1, the combination becomes more powerful at turning genes on and off," says the study's first author, Dr. Hayriye Verda Erkizan, a postdoctoral researcher in Toretsky's lab who is presenting the study results at AACR.
Using a lab method of keeping RHA apart from the fusion protein revealed that both were important to cancer formation, and thereafter the researchers decided to explore molecules that could keep the two proteins separated.
The researchers used a library of small molecules loaned to Georgetown from the National Cancer Institute, and tested 3,000 compounds. They found that one of them could very tightly bind to immobilized EWS-FLI1 proteins.
Erkizan said that the discovery was wonderful because scientists had always thought that it was not possible to block protein-protein interactions, as the surface of the proteins are slippery and much too flexible for a drug to bind to.
"These are wiggly proteins yet this study shows that inhibition of protein-protein interactions with a small molecule is possible," Toretsky says.
This possibility means that fusion proteins, such as those produced in other sarcomas as well as diverse disorders, might be inhibited, he says.
Source-ANI
RAS/L