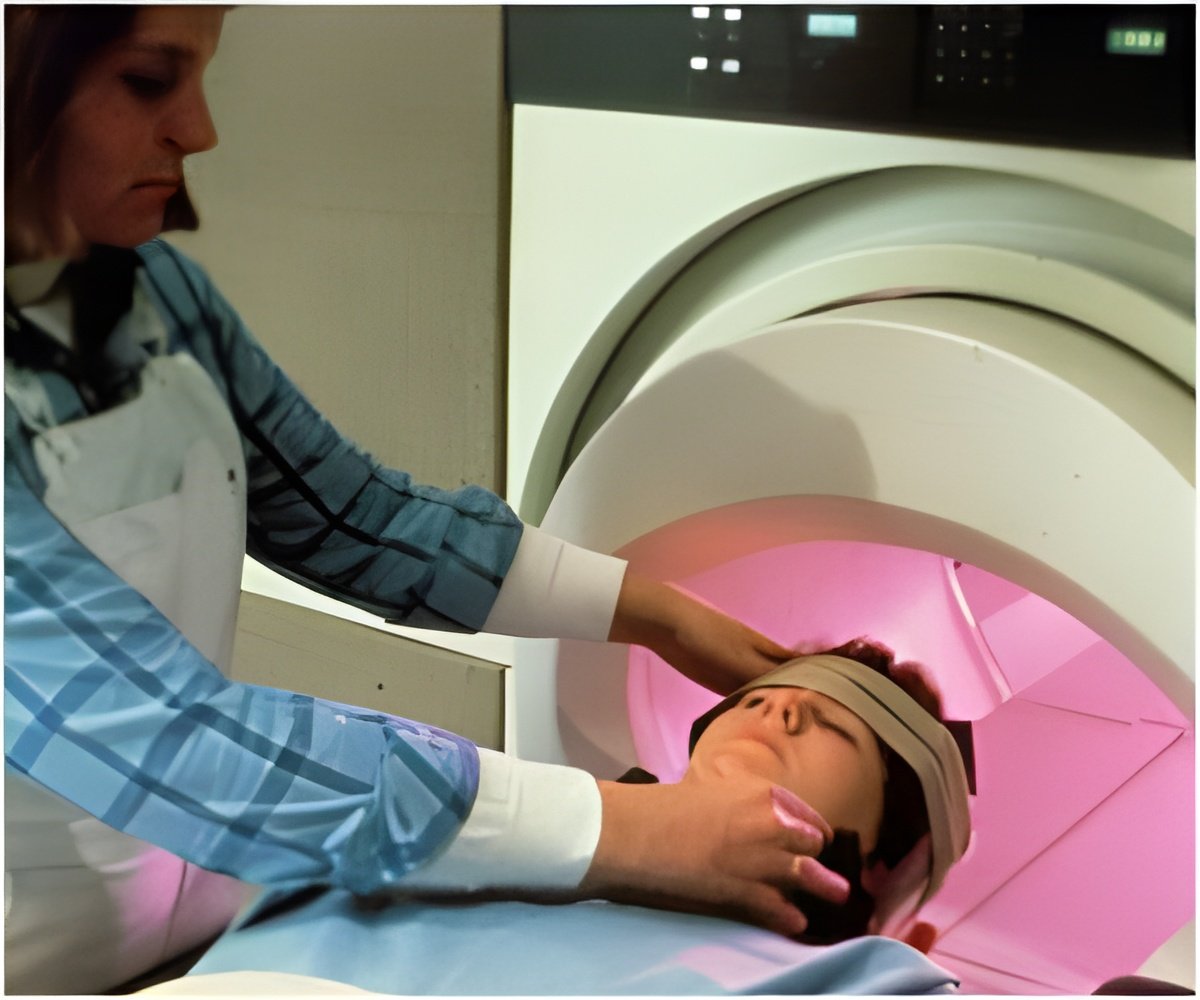
The new approval allows patients to receive MRI scans of any part of the body, as long as specific precautions are taken by the radiology technicians.
These are the only DBS systems to receive a full-body MR-conditional approval from the European authorities. The system allows patients with Parkinson’s disease, essential tremor, refractory epilepsy, and dystonia to continue receiving medical care that requires MR scans.
Medtronic press release states that the implants have to be appropriately programmed before undergoing a scan. This will help the acquisition of sharp images unaffected by the jerky motions associated with neuromotor disease.
Source-Medindia