Macular degeneration treated with cancer drug Avastin has led to a spike in eye inflammation in Canada.
First noticed in British Columbia, there appears to be a rise in cases in other parts of the country as well.Public health authorities are investigating the phenomenon and also trying to draw the potential problem to the attention of the global public health community in the hopes of seeing if it is being observed elsewhere.
The problem is believed to stem from a particular lot of the drug that was distributed widely around the world, though not to the United States. The lot number is B3002B028.
The drug's manufacturer, the Swiss pharmaceutical firm Roche, is co-operating with Health Canada and the British Columbia Centre for Disease Control, which was alerted to the problem by ophthalmologists.
The situation puts Roche in an awkward position. Avastin (bevacizumab) is a colon cancer drug; it is not approved for use as a treatment age-related macular degeneration.
And Roche doesn't want to encourage the off-label use of the drug by ophthalmologists who have embraced it as a much cheaper alternative to a similar drug, Lucentis, which has been approved as a treatment for macular degeneration.
Advertisement
Ouimet said the company is in discussions with Health Canada. But it is reluctant to put out an advisory warning people not to use the drug as a treatment for age-related macular degeneration when the drug was never approved for this purpose.
Advertisement
"Is it a delicate situation? Absolutely."
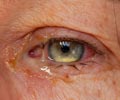
Ophthamologists in British Columbia started noticing a problem in October. Under two per cent of people treated for age-related macular degeneration with Avastin will develop acute intra-ocular inflammation. But between Oct. 3 and Oct. 27, the rates were much higher.
The BC CDC was called in to help investigate the cluster of cases, and concluded the rate of inflammation among patients treated with Avastin from the lot in question was almost 10 times higher than the normal rate. The lot is no longer in use there.
Dr. David Patrick, the centre's director of epidemiology services, submitted a report on the investigation to ProMED, an electronic reporting system that sends out alerts about outbreaks to a mailing list of public health officials, scientists and other interested parties around the world.
Patrick said the team investigating the issue sent out feelers to ophthamologists in about a half-dozen other Canadian centres and has heard back that others too have noticed an increase in cases of inflammation after use.
The inflammation causes cloudy vision, but appears to clear up over time, he said.
Ouimet said there have been no reports of problems from other countries - and no reports of adverse reactions in cancer patients who received treatments from the same lot.
"We have reviewed all the analytical release data for the lot in question. And all the best parameters were within the limits for use in oncology," she said.
"So we found no deviations in the manufacturing process. All the environmental testing was fully compliant. We've revisited the batch. It is safe … for its indicated purpose."
Patrick said scientists at the University of British Columbia are studying vials of the drug, but haven't found anything usual.
"On the initial go-through they haven't determined a chemical difference between the implicated lot and another one. But there may well be further work with that," he said.
Source-Medindia
GPL/SK