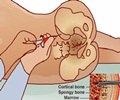
All patients who received the treatment tolerated the procedure well without any immediate serious adverse effects. Thirty-three percent of treated infants developed moderate BPD and none developed severe BPD, and 72 percent of a matched comparison group developed moderate or severe BPD. Another serious side effect of very preterm birth, retinopathy of prematurity requiring surgery, tended to occur less often in treated infants. Overall, all nine treated infants survived to discharge, and only three developed moderate BPD.
This phase I study suggests that intratracheal administration of mesenchymal stem cells is safe and feasible. According to Dr. Park, "These findings strongly suggest that phase II clinical trials are warranted to test the efficacy of mesencymal stem cell transplantation, which could lead to new therapies to prevent or cure BPD." Dr. Park and colleagues are currently conducting a long-term safety and follow-up study of these nine preterm infants ClinicalTrials.gov: NCT01632475).
Source-Eurekalert