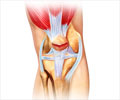
The US Food and Drug Administration revealed that Johnson & Johnson has issued a recall notice for its knee replacement device following reports that it increased the risk of fractures.
The device, known as LPS Diaphyseal Sleeve, is manufactured by J&J’s subsidiary, DePuy Orthopaedics, and is used in patients who receive knee replacement with the company's LPS System. Terming the recall as class one, the most serious type of classification, the FDA said that the device has a high potential of fracture which could lead to loss of function or even death.
“The LPS Diaphyseal Sleeve Base taper connection may not be sufficient to accommodate potential physiologic loads that may be transferred to the junction during normal gait activities by some patients. This may result in fracture of the sleeve at the taper joint which may also lead to loss of function or loss of limb, infection, compromised soft tissue or death”, the FDA statement said.
Source-Medindia