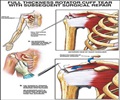
‘CBD is safe and effective in reducing pain in the immediate peri-operative period following rotator cuff repair and should be considered in postoperative multimodal pain control.’
Tweet it Now
Currently, Cannabidiol is FDA approved for the treatment of some seizures and other studies have suggested the therapy may help treat anxiety, cognition, and movement disorders though research has yet to prove it can be used to treat these disorders. Patients were followed-up on days one, two, seven, and 14, and the Visual Analog Scale (VAS) for pain, opioid consumption, and satisfaction with pain control were recorded. The VAS is a validated, subjective measure for acute and chronic pain. Scores are recorded by making a handwritten mark on a 10-cm line that represents a continuum between “no pain” and “worst pain.” Additionally, liver function tests (LFT) were done on days seven and 14 to assess safety, and nausea was monitored.
On day one, the VAS pain score was significantly lower in those receiving CBD (4.1 +- 3.0 vs 6.0 +- 3.1, p = 0.01), which also meets VAS MCID for shoulder arthroscopy (1.2-1.4). Patient satisfaction with pain control trended towards significance favoring the CBD group (72% vs 60%, p = 0.072). However, there were no statistically significant differences in opioid consumption (16.4mme +- 12.7 vs 20.6mme +- 14.4, p = 0.186), which was relatively small in both groups.
Notably, patients receiving 50 mg of CBD reported lower VAS scores at day 1 (3.9 +- 3.3 vs 4.6 +-2.5 +- 6.0 +- 3.1, p = 0.0394) and higher satisfaction with pain control at day 1 and 2 (8.1 +- 2.3, 5.8 +- 3.3, 5.9 vs 3.6, p = 0.0175 | 7.8 +- 1.8 vs 6.3 +- 3.0 6.0 +- 3.2, p = 0.05) compared to those receiving 25 mg of CBD and control group respectively. On day sever and day 14, there were no statistically significant differences in VAS score, opioid consumption, or patient satisfaction with pain control (p>0.05 for all). There were no significant differences in nausea or LFTs post-operatively (p > 0.05).
Advertisement