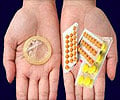
Indian authorities have threatened to pull advertising for two brands of morning-after-pills off the air. The reason? The ads promote the drugs as regular contraceptives and misrepresent abortion.
The manufacturers of "Unwanted 72" and "I-Pill" have launched an advertising blitz on satellite TV channels in recent months pushing the morning-after-pills as a means to be "tension free" after sex.The campaign for I-Pill shows a terrified young woman cowering in the backseat of a taxi ferrying her and a female friend to an abortion clinic, a health ministry official said.
"We have ethical concerns about these advertisements, they are not projecting the message clearly that these pills are emergency contraceptives and not regular contraceptives," Ram Teke, the deputy Drug Controller General of India (DCGI) told AFP Wednesday.
"The language too is objectionable -- one advertisement says that couples can be 'tension free' if they have access to emergency contraception. This is not correct," Teke said.
"We are awaiting the companies' response. This office (DCGI) gave them the permission to advertise and if they do not take corrective action, the campaigns can be pulled off air."
Morning-after-pills were made available just a year ago after the government gave pharmacists permission to sell them over the counter "to give women rights over their sexuality and fertility," the health ministry official said.
Advertisement
The health official, who preferred not to be named, said the depiction of abortion was a cause for concern.
Advertisement
Teke said the directions to the manufacturers were issued after feedback was gathered from doctors and gynaecologists.
Source-AFP
TAN