Results of the phase 3 NETTER-1 study, showing results for Lutathera in patients with metastatic midgut neuroendocrine tumors, will be presented by researchers.
‘Progression-free survival (PFS) for patients treated with Lutathera was improved than patients treated with Octreotide. The safety profile of Lutathera was also favorable.’





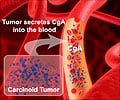
Lutathera is a radiolabeled (Lu-177) somatostatin analogue that functions similar to standard somatostatin analogs, but also delivers toxic radiation directly to the tumor. Lutathera has received orphan drug status from the European Medicines Agency and the U.S. Food and Drug Administration. Moffitt Cancer Center will present results of the phase 3 NETTER-1 study, showing clinically meaningful and significant results for Lutathera (77Lu-DOTA0-Tyr3-Octreotate) in patients with metastatic midgut neuroendocrine tumors. The data will be presented Monday during the American Society of Clinical Oncology Annual Meeting in Chicago.
The NETTER-1 phase 3 trial compares Lutathera to Octreotide LAR in patients with inoperable midgut carcinoid tumors that progressed following Octreotide LAR and express the somatostatin receptor.
Results to date in 230 randomized patients show improved outcomes for patients treated with Lutathera over Octreotide LAR. Median progression-free survival (PFS) was not reached in the Lutathera arm and was 8.4 months in the Octreotide LAR arm (P < 0.0001, HR 0.21), while the objective radiographic response rate (ORR) was 19% for Lutathera treated patients and 3% for Octreotide treated patients (P = 0.0008).
The safety profile of Lutathera was also favorable, with only 5% of patients experiencing a toxicity that led to a dose modification. Grade 3 or 4 adverse events were low, with the most common being neutropenia (1%), thrombocytopenia (2%) and lymphopenia (9%).
Advertisement