‘The finding is a significant breakthrough that will lead to better treatments of the genetic disease.’
Tweet it Now
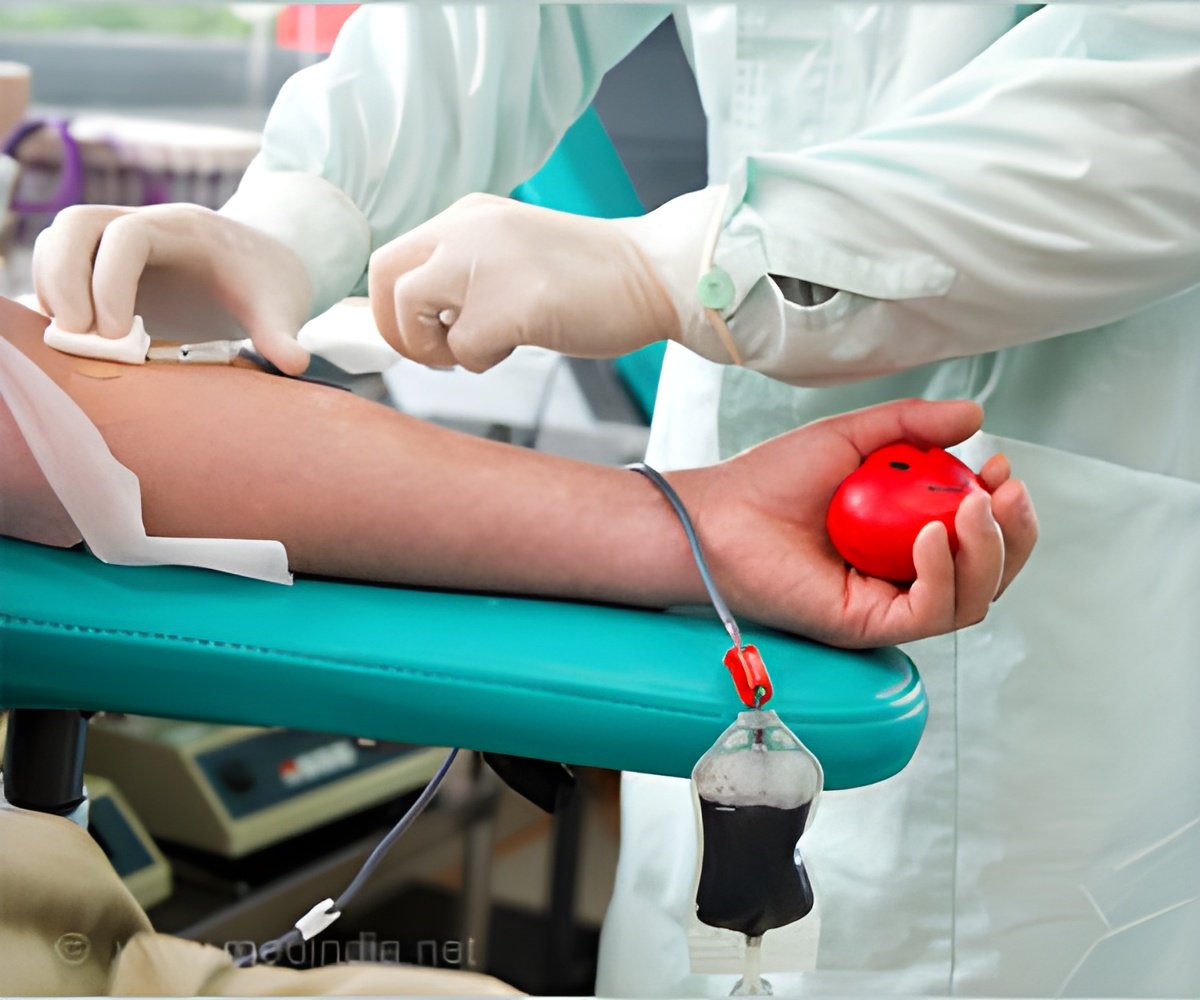
"The current production of Alpha-1-antitrypsin from blood requires a constant and large supply of blood from donors. With a recombinant alternative, you can both formulate the product, concentrate the protein, purify it, and avoid dependency of blood donors - so we would be able to ensure security of supply," says Bjørn Voldborg, Director of CHO Cell Line Development at The Novo Nordisk Foundation Center for Biosustainability. CHO-cells can produce over 1 gram
The researchers have successfully produced Alpha-1-antitrypsin in CHO cells - one of the most deployed cell lines for production of human therapeutic proteins. Normally, the CHO glycosylation profile of Alpha-1-antitrypsin is quite different from the human counterpart. The human version of Alpha-1-antitrypsin has a very specific sugar-structure, whereas if produced in CHO cells exhibits a variety of sugars, which renders the protein ineffective in the human body.
The researchers obtained this glycosylation profile by introducing a human gene encoding an enzyme capable of decorating the sugar backbones on the Alpha-1-antitrypsin proteins with the correct human moieties. Furthermore, they removed a number of the CHO cells natural glycosylation genes using CRISPR-Cas9.
This effort resulted in a cell line capable of producing over 1.2 grams pr. liter of cultured CHO cells, thereby making it possible to produce Alpha-1-antitrypsin from CHO cells at a scale that can eliminate the need for human donors in the future.
Advertisement
Today, CHO-cells with the best yield produce around 10-12 g/L of monoclonal antibodies. Hence, this Alpha1-antitrypsin producing cell line is still only producing around 10 % of this known maximum. One of the next steps is, therefore, to improve the yield even further.
Advertisement
The natural Alpha-1-antitrypsin is being degraded rather quickly in the body. Going forward, researchers wants to make the recombinant proteins more stable, so Alpha1-antitrypsin deficiency patients would need fewer injections. Hence, this new knowledge about glycosylation profiles opens a new window for optimizing human proteins to improve their stability and function.
"Our hope is to optimize stability and to become so good at designing glycans, that researchers and companies can order glycan structures on any protein from us. This will accelerate the production of new glycosylated proteins for therapeutic use," says Bjørn Voldborg.
Source-Eurekalert