- A new technology which involves a collection, storage, and transport system has now been approved by the USFDA. It is a paradigm shift in tuberculosis (TB) sample transport technology
- The tuberculosis bacterium, M.tuberculosis is inactivated and its RNA/DNA stabilized for safe, efficient, and reliable sample transportation
- Safe transport ensures the reach of the diagnostic tool to remote places in the world thus helping in the worldwide fight against tuberculosis.
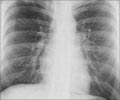
TB is highly infectious, and the bacterium shows resistance to many or all current antimicrobials. Hence, care must be taken while transporting the live infectious samples so that we do not facilitate the spread of unknown pathogens from one region to another. The pathogens in the samples can be rendered safe if they are inactivated; the RNA and DNA also need to be stabilized to provide safety at all stages of the transport and testing process from a healthcare worker to a laboratory technician safety personnel. RNA and DNA stability also allows a single sample to be tested multiple times for TB as well as for other respiratory pathogens that can simultaneously infect TB patients.
"We are excited to announce the FDA clearance of PrimeStore®MTM, a paradigm shift in biological sample transport technology. Leaders of the TB community are driving the rapid phase out of centuries old diagnostic methods, and fully embracing current and evolving technologies in molecular diagnostic testing, next-generation sequencing, digital radiology, and software including DNA analysis and artificial intelligence," states Jeff Fischer, President of Longhorn Vaccines and Diagnostics LLC. "Industry competition which relies on quality peer- reviewed science, and trusted, transparent, and unbiased regulatory review such as the US FDA 510(k) process is essential to the rapid adoption of emerging technologies and maximizing the value of government and donor funding to truly make a difference for those that are and those that will be afflicted with this terrible, highly curable disease."
About Primestone MTM – single step formulation and an improvement over other collection and storage solutions
A collection, storage, and transport system that inactivates and lyses biological species like viruses and bacteria including M. tuberculosis while maintaining the integrity of the nucleic acids (RNA/DNA), all in a single collection vessel.- Biological components are inactivated, nucleic acids are separated and released through lysis of cells, and integrity of RNA/DNA is maintained more or less simultaneously.
- RNA/DNA integrity is preserved for prolonged time periods through multiple freeze/thaw cycles. Specimens can be stored or transported frozen, refrigerated or at ambient temperatures.
- Sputum samples can be stably transported over days to months and moved from location to location at ambient temperatures, anywhere in the world; this improves access to timely and sensitive TB diagnostic testing.
- Diagnostic and molecular testing can be done at the point of care, like in doctor’s offices, clinics, and emergency departments; inactivating the samples ensures that healthcare workers are not exposed, to expected or unexpected pathogens.
- Samples can be tested multiple times - negative specimens can be transported for further testing, and positive tests can be sent to a higher-level laboratory for further characterization.
- Compatible with manual and high-throughput extractions systems and PCR systems run in hospitals and centralized laboratories.
- Sample sputum amount required can be as little as 1/10 the sample amount used in standard procedures thus overcoming drawbacks of standard kits that require larger sputum sample sizes.
Reference:
- A clinical specimen collection and transport medium for molecular diagnostic and genomic applications - (https://www.researchgate.net/publication/49727970_A_clinical_specimen
_collection_and_transport_medium_for_molecular_diagnostic_and_genomic_applications)